Flameless AAS provides a means to increase flame AAS sensitivity, by up to 200 times. Interferences from aqueous leachates and digestates are so great, however, that flameless AAS has found application mainly after organic extraction. Thereby, the analytical sensitivity is increased far beyond that of the SX/flame- AAS methods.
Interferences
Interferences from other elements have been reported as minimal in many cases, but matrix difficulties and precautionary steps to avoid them are also prevalent in the literature (see Mallett [1970] and Sen Gupta, [1973]). High salt content, from some elements more than others, causes AAS interferences from broad band spectra and light scattering—i. e., Fe3+, NaCN, KCN, Zn, Se, Te, Ni, Co, and Cu in the case of gold and Fe3+, NaCN and KCN in the case of silver. Unless the salts are very concentrated, e.g., as when several grams of soluble sample are dissolved in 100 ml, we have found that background correction eliminates such interferences. Without background correction, we have found that quantitative results are difficult to attain. As reliable background correctors were not common until the late 1960’s, it may be assumed that many problems reported were, in fact, due to the lack of this important AAS accessory. Even when a background corrector is used, we strongly recommend that its functionality be tested frequently with a matrix blank which matches the sample. This should read zero if the background corrector is working.
At very high salt concentrations, the background corrector often cannot do its job; the high viscosity of concentrated solutions also impedes aspiration. In the extreme, salts may collect on the burner head. Although complexing agents have sometimes been added to increase solubilities of some species, solvent extraction appears the more common remedy. Even then, some species, e.g., Fe, may extract into the organic, rendering its properties unsuitable for aspiration. For this reason, the MIBK is given several washes with dilute (e.g., 1.5 M) HCl to remove interferent, the gold remaining in the organic phase.
Determination of Gold and Silver from a Single Sample Preparation
Gold and silver are often determined from the same sample, so a single sample preparation prior to AAS measurement offers substantial time savings. Single sample preparation is of course, easily accomplished when both metals dissolve in the digest (e.g., aqua regia) or the leach solution (e.g., cyanide) at levels measurable with flame AAS.
Often the gold/silver ratio dictates that gold must be extracted but that silver can be determined directly from the digest. Tindall (1965) accomplished this from an aqua regia digest with 25% HCl makeup, and solvent extraction of gold into MIBK. Silver was determined from the HCl before the gold extraction (this is necessary as silver extracts incompletely into the MIBK), and gold was determined from the MIBK. The gold standards were prepared by extraction into the ketone from 25% HCl.
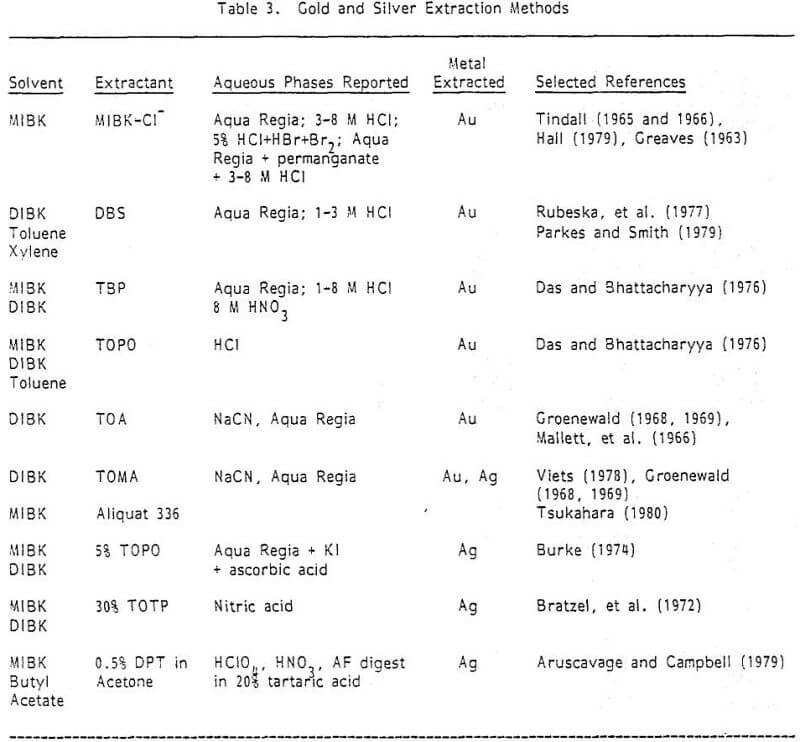
It would be advantageous to extract both the gold and silver from a common lixiviant (e.g., aqua regia), and aspirate them from a common organic solvent. This has been done (see Konovalov and Gureva, 1979) for high levels of Au and Ag in Sb-Pb alloys using petroleum sulfides in toluene as the extractant. Considering flame characteristics of this solvent, it would appear more suitable for ores to use some of the extractants in Table 3 which are common to both silver and gold—e.g., TOPO in MIBK or Aliquat 336 in DIBK. Such a method could not be found in the literature. It also appears possible to dissolve these metals, after aqua regia digestion and baking, with a NaCN solution and to either extract both metals in Aliquat 336/DIBK from that solution or directly aspirate the silver and extract the gold.