The flocculation behavior of coal, quartz and clay was studied by measuring the turbidity of the supernatant liquid after one hour of settling. Figure 14 is a plot of turbidity and adsorption density against equilibrium concentration. The turbidity of the supernatant liquid decreases until a minimum is reached and tends to rise thereafter. The results are consistent with the bridging theory which stipulates that the polymer molecule attaches itself to one or more adsorption sites; the remainder of the molecule extending into the solution, free to interact with vacant sites on another particle. As the polymer concentration increases, fewer sites are available for adsorption and the extended segment is less able to adsorb on the surface of another particle. The flocculation, then, deteriorates because of the formation of a protective coating of polymer on the particle surface adding its own charge to that of the particle. Further addition can in fact lead to stabilization of the suspension. It can be seen from the figure that the optimum dosage for coal using polymer A is reached at less than one-sixth coverage of the surface and that the performance of the polymer deteriorates once the optimum dosage is exceeded. The flocculation performance of polymers A and B was studied under a wide range of conditions; the results are presented below.
Effect of pH and Ionic Strength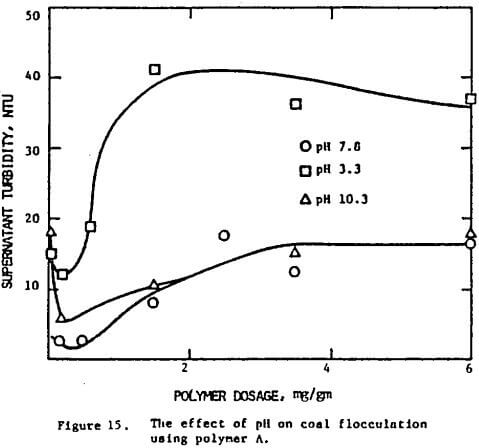
Figure 15 indicates that the optimum dosage for a coal suspension is more or less constant regardless of the pH. Similar results were found in the case of ionic strength. However the clarity of the suspension does depend on these two variables. The lowest turbidity reading was obtained at pH 7.8. In all cases the flocculation was found to get poorer when the optimum dosage was exceeded, although the deterioration was much less when the suspension was at high ionic strength.
Again, the pH effect can be explained in terms of the configuration of the polymer molecules. At neutral and alkaline pH, the molecules are extended in solution, thereby favoring the establishment of polymer bridges between particles. Under acid conditions, the molecules are tightly coiled, bridging is less likely to occur, and flocculation is found to be less effective. The correlation can be readily seen in Figure 18 where the minimum turbidity and the radius of gyration are plotted against pH.
For the quartz and clay systems, the flocculation seems to be controlled by changes in surface charge rather than by polymer configuration. The most efficient flocculation is obtained at acidic pH where the charges on the surface are small.
As the pH increases, the flocculation becomes poorer. When salt is added to these suspensions, the double layer is compressed and flocculation by bridging is favored because particles can approach one another more closely.
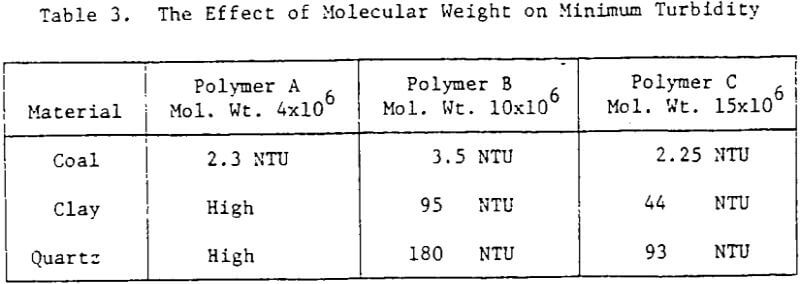
Effect of Molecular Weight
No change in the optimum dosage was observed with molecular weight. However as shown in Table 3, there is a definite improvement in the performance of the flocculants as molecular weight increases, especially for the clay and quartz suspensions.