First let’s change the amount of the Flotation Depressants used. The valuable mineral that isn’t liberated from the unwanted mineral will begin to be depressed along with the waste. For the floatation operator, the change that will become most apparent is, that the color of the froth will begin to reflect more of the wanted mineral’s color. 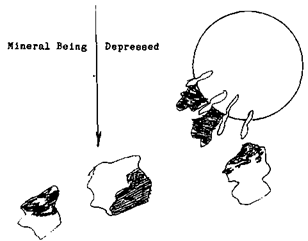 This is because the froth is becoming purer as the unwanted mineral, the iron pyrite, is being cleaned off of the bubble.
This is because the froth is becoming purer as the unwanted mineral, the iron pyrite, is being cleaned off of the bubble.
Not only is the colour of the bubble changing but the size is as well, the weight that the bubble has to support is lessening as the impurities and un-liberated (locked) valuables are depressed. This of course causes the bubble to expand in size. When the bubble reaches the surface it will be large and unstable. The more mineral that is depressed the larger the bubble will become until there isn’t enough weight on the bubbles surface to prevent it from breaking.
As we continue along with this lesson you will notice that more than one reagent flow will produce similar reactions in the shape size and colour of the bubble. With experience and carefully monitoring reagent addition rates you will come to know which reagent requires adjustment by simply looking at the flotation cell.
 O.K. Now! Let’s look at what happens with TOO LITTLE DEPRESSANT being added. First the collector begins to pick up more particles of mineral. Which of course means more of the impurities that had been depressed. The froth begins to look more like the unwanted mineral than it should. The bubbles will have to carry more of the mineral that means they will begin to get smaller as the extra weight compresses them. Also the extra particles will overload the collector molecules. This will make the bond between the molecule and the bubble as well as the bond between the particle and the molecule weak. Both will begin to fall free of the bubbles. As these particles that do fall off descend into the cell they become trapped by the tighter smaller bubbles that are caused by the pressure of the slurry in the cell. This gives the bubble extra weight to try and expand against. As the weight increases the bubble quits forming properly. This interferes with the flow of froth over the side of the cell and the cell becomes overloaded. As the overload becomes worse the overflow slows down and recovery drops.
O.K. Now! Let’s look at what happens with TOO LITTLE DEPRESSANT being added. First the collector begins to pick up more particles of mineral. Which of course means more of the impurities that had been depressed. The froth begins to look more like the unwanted mineral than it should. The bubbles will have to carry more of the mineral that means they will begin to get smaller as the extra weight compresses them. Also the extra particles will overload the collector molecules. This will make the bond between the molecule and the bubble as well as the bond between the particle and the molecule weak. Both will begin to fall free of the bubbles. As these particles that do fall off descend into the cell they become trapped by the tighter smaller bubbles that are caused by the pressure of the slurry in the cell. This gives the bubble extra weight to try and expand against. As the weight increases the bubble quits forming properly. This interferes with the flow of froth over the side of the cell and the cell becomes overloaded. As the overload becomes worse the overflow slows down and recovery drops.
For the operator his clues are a change in colour, reflecting more of the unwanted mineral. The size of the bubble, getting larger in the beginning, deteriorating to a tight froth with small bubbles and a visible portion of the mineral sinking.
Depressing Reagents
The commonest depressing reagents are lime, sodium cyanide, and zinc sulphate. Lime is extensively used to prevent pyrite from entering the concentrate of other minerals particularly copper and zinc sulphides. Its action is positive and progressive ; it is only necessary to add it to the pulp in sufficient quantity to make the pyrite unfloatable. The other minerals, are depressed at the same time, but can be floated again by the addition of sufficient xanthate, which will have no effect on the pyrite provided that enough lime has been added. If the quantity of reagents needed be prohibitive as regards cost a compromise is necessary ; the lime addition is balanced against that of the xanthate in order to give a profitable recovery of the valuable minerals in a concentrate of the required grade without too much pyrite.
The same method holds good for making a clean concentrate of sphalerite in the second stage of the separation of lead from zinc minerals in a pyritic ore. Here lime is not usually employed in the first stage, where the sphalerite is depressed by sodium cyanide and the galena brought up by carefully controlled additions of ethyl xanthate or thio-carbanilide, because the cyanide generally keeps down the pyrite as effectively as it does the sphalerite. Should lime be required, however, to depress pyrite in the first stage, one of the more powerful higher xanthates will be necessary to give a good recovery of the galena.
Sodium cyanide
Sodium cyanide is mainly used to depress sphalerite in the first or lead-flotation stage in separating lead from zinc minerals. Its action on pyrite is nearly as strong as on sphalerite, but it does not affect galena unless a large excess is added or the contact time is too long.
The effect of sodium cyanide is often intensified by the addition of zinc sulphate. The crystalline salt ZnSO4.7H2O is usually employed, although to save freight charges the lower hydrate ZnSO4.2H2O may be substituted. While no fixed rule can be laid down regulating the proportion of sodium cyanide to zinc sulphate for most efficient work, it is generally found that, for a given ore, the ratio of reagents can be kept constant, although the total quantity used may have to be varied from time to time.
Zinc sulphate
Zinc sulphate does not always intensify the action of the cyanide, the latter often being just as effective by itself ; there are also cases where the former alone gives better results. The most effective reagent or combination can only be found by experiment.
The consumption of sodium cyanide normally ranges from 0.1 to 0.3 lb. per ton of ore and that of ZnSO4.7H2O from 0.3 to 0.9 lb. per ton. These are added to the circuit in the form of solutions containing about 20% of the solid.
Sodium cyanide is occasionally substituted for lime in the flotation of a sulphide copper ore containing pyrite, since it has a much greater depressing effect on the pyrite than on the copper minerals. Xanthate is generally employed to bring up the copper sulphides, its addition being balanced against that of the cyanide to effect a profitable recovery of the copper with the minimum amount of pyrite.
Sodium sulphite was at one time employed as a depressor of sphalerite, its action being much the same as that of cyanide but less effective. Its use is seldom encountered, except in instances where cyanide has been found too powerful.
