Differential and preferential flotation
The pressing problems in differential and preferential flotation of ores are (1) the separation of lead sulphide from zinc sulphide, and (2) the separation of zinc and copper bearing sulphides from sulphides of iron.
Methods seeking separation of one sulphide from another by flotation are of two classes, viz.: (1) those in which a difference in flotability of the two minerals is created or enhanced by conscious control of one or more of the activating phenomena of the process, and (2) those in which the chemical nature of one of the sulphides is changed, with a resulting change in the nature of the surface. Methods of the first class truly accomplish “differential flotation” and this denomination should be closely restricted to such methods. Methods of the second class, by preferential chemical action, so change one of the sulphide minerals that it never floats, leaving the unaltered mineral to float under proper conditions. Freeman (Min. and Sci. Press, June 5, 1920, abstract from Proc. Australasian Inst, of M. and M.) calls this “preferential flotation” and this terminology may profitably be adopted.
Differential flotation of galena in the presence of blende may be brought about, in pulp-body concentration, by proper control of gasification and of kind and quantity of “oil,” in the presence of fresh water. This merely means that as the conditions under which gas is precipitated from the water are gradually brought up to the point where maximum precipitation occurs, a point is first reached where the gas precipitates preferentially on the galena. Gas precipitation is increased by agitation, by acid, and by heat, and the necessary adherence of the gas bubbles after precipitation is aided by the presence of oil on the sulphide particles. While there is no direct evidence on the point, there is indirect evidence pointing to the fact that oiling of galena takes place under given conditions more rapidly than corresponding oiling of blende. Hence by using a very small amount of oil with, in the agitation-froth process, a relatively gentle agitation, differential oiling of the galena probably occurs. And with the same relatively gentle agitation and in the cold, differential precipitation of gas on the oiled galena occurs, resulting in flotation of galena and non-flotation of the blende. Increase in agitation or application of heat or addition of acid or two or all of these accompanied by the addition of more oil, either of the same or a different kind, will result in raising blende.
The differential flotation effect above noted and described is greatly enhanced by the presence, in acid solution, of certain salts, chiefly sulphates; or by sulphur dioxide (SO2) in an otherwise non-acid pulp; or, it is said, by the presence of sulphur in solution; or by a permanganate; or soda ash. When sulphates are used in the flotation of the galena, subsequent flotation of the blende is brought about by a marked raise in temperature and by the addition of more acid and oil; where SO2 is used it is driven off after the galena flotation by heat or aeration or is chemically neutralized, and acid and more oil are added; with “sulphur in solution” and with permanganate, oil, acid and heat are added to accomplish the blende flotation; with soda ash more oil and a salt, such as copper sulphate, produce the blende flotation.
It is distinctly in favor of the methods that float galena away from blende that, since associated silver usually floats with whichever mineral is first floated, silver in a lead concentrate is much more valuable than silver in a zinc concentrate.
“Preferential flotation” of blende from a mixture of blende and galena is accomplished by altering the galena through chemical reaction. This has been done by a low-temperature oxidizing roast, which changes the galena, at least superficially, into the non-lustrous sulphate; or by digesting the feed with a hot acid solution of ferric chloride; or with bichromates; or with “acid salts.”
It is to be especially noticed in connection with these processes that, since the altered galena is non-floatable, if separation of lead from gangue is to be effected, a mixed float of lead and zinc minerals must first be made and this concentrate then be chemically treated and again subjected to flotation to separate the blende. This treatment leaves a lead concentrate as the underflow from the flotation machine, where otherwise a worthless leady-tailing would be discharged.
Separation of copper-bearing sulphide from iron sulphide has been worked out on a laboratory scale by using a deficient amount of a flotation agent, which, when present in greater amount, will float both of the sulphides, although even under such circumstances exhibiting some differential action. No one flotation agent possesses this differential characteristic. Each such case is, in the present state of our knowledge, an individual one which must be investigated, having in mind the cardinal principle.
Sampling
In laboratory work sampling is simple, if the batch to be tested is small and ground dry. In such case an assay sample of the feed should be riffled out of the lot, and the products of each test should be dried and weighed and samples for assay then taken by riffling. With a known weight of feed a check is thus obtained on the accuracy and care of the testing work and on the accuracy of the sampling and assaying.
When the sample for laboratory testing is a wet pulp, sampling the feed is a difficult matter. Practice in the writer’s laboratory is to get the solids in suspension as completely as possible by stirring and then to take duplicate samples by dipping out two 300-cc. beakerfuls of pulp, plunging the beaker quickly well beneath the surface of the pulp. These samples are then dried, riffled and assayed. Concordant results are taken to indicate accuracy.
In a test mill the sampling comprises both tonnage and assay samples. Sampling of feed, tailing and concentrate for assay should be automatic. Feed samples will be more nearly representative the finer ground and more fluid the pulp sampled. Hence where possible the material sampled should be the actual flotation feed just before it enters the flotation machine. Provision should be made at this point also for tonnage sampling. This may be by volume or weight as desired, either method requiring the simultaneous taking of a moisture or pulp-density sample. For tonnage measurements by volume a container, large enough, if possible, to take the total flow for a minute, should be provided, and the launder carrying the pulp to be sampled should be so arranged that the whole flow can be quickly diverted into the container and subsequently as quickly away. If oil is present in the pulp to be sampled the container should be calibrated to overflow, as the froth which forms on the pulp in the container would mask any other end point. When the tonnage sample is weighed, the container should be set on a pair of platform scales, the requirements as to size of container and pulp diversion being the same as before. Now, however, no requirement as to overflowing obtains.
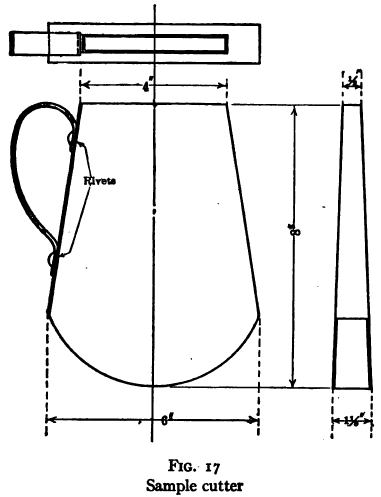
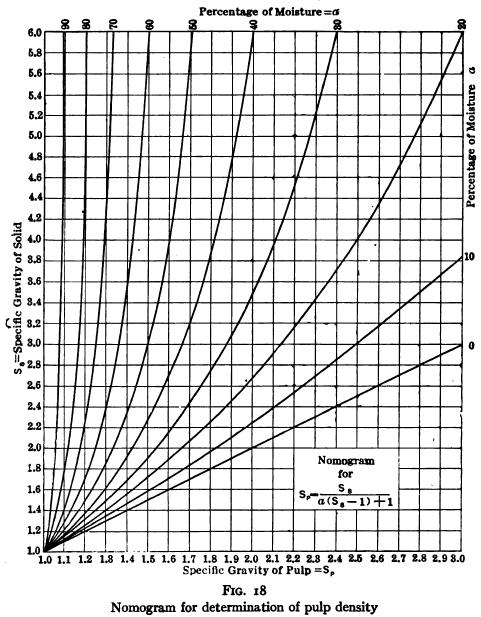
Pulp densities are obtained from the determination of the weight of a small sample of known volume. This should be cut from the sample stream at the time that the tonnage sample is being taken, by means of a slotted cutter of the type and proportions shown in Fig. 17, and should be washed into a tared, wide-mouthed measuring container by means of a measured volume of water. Then, knowing the specific gravity of the ore, the percentage of solids and hence the weight of solids in the sample can be computed by formula (see page 163) or can be obtained from the chart, Fig. 18.
Preparation of ores for tests
The preparation of ores for flotation tests depends upon the kind and purpose of the test to be made. In general the common sense of the operator is a sufficient guide. Thus, if the test is a laboratory test made to determine the reason for some mill performance, the feed pulp will be prepared in the mill itself and the sample will be taken, of course, at the head of the mill flotation machine. But if the test is a laboratory test on an ore to determine, for instance, the amenability of the ore for flotation, it is, in general, best to grind the ore dry for all of the preliminary testing work. The usual machines employed are a small crusher to bring the ore to, say, minus-1/4 or minus-3/16-inch; a pulverizer to further reduce it to pass, say, a one-millimeter screen; and finally a laboratory pebble mill. Such a method of reduction produces a pulp somewhat different from that produced by ordinary wet-grinding mill methods. If all the pulp is dry-ground through some limiting screen, a greater proportion will pass a 0.074-mm. screen than would be the case with a wet-ground pulp in the mill where the same limiting upper size is sought. This difference will tend to cause the results with the dry-ground ore to be better than would be obtained on the mill pulp. On the other hand, if the mill grinding is to be done in the presence of the flotation agent, the favorable effects of this treatment on subsequent flotation will probably more than offset the advantage to the laboratory test of the finer grinding. It may be set down also, as a general tendency, that, all other conditions being as nearly as possible equal, finer grinding is necessary for a laboratory test than for mill operations, if the same result is to be obtained.
Nomogram for determination of pulp density
In general, as regards the storage of feeds for testing purposes: Selection in flotation depends entirely upon the nature of the surfaces of the solid particles in the pulp. It is probably not strictly accurate to say that the surfaces of the selected solids must be clean and of a truly metallic, adamantine or resinous luster, but the behavior of samples under certain conditions in flotation testing indicates that some such requirement does exist. It is a fact that a sulphide ore, which has been so treated as to induce oxidation of the sulphide minerals or coating of these minerals with any sort of a solid or semi-solid covering is entirely changed insofar as flotation character is concerned; thus a sample of pulp cut at the head of a machine in which successful flotation is being carried forward, may, and usually will, if allowed to stand for a considerable length of time, or if evaporated to dryness and again wet, partially or utterly fail to give any satisfactory concentration by flotation, if treated under conditions in the laboratory machine otherwise similar to conditions in the mill. Similarly a dry sample that is allowed to stand around a laboratory in which there is any considerable amount of acid fumes, may, after a considerable length of time, show entirely different flotation characteristics from those exhibited by the fresh sample. Ores ground to a given size in a pulverizer of the Braun disk type, may show an entirely different behavior in a flotation test from another sample from the same lot, ground dry in a laboratory pebble mill to pass the same limiting screen, and the dry-ground samples in both cases may show an entirely different flotation performance from one wet-ground in a laboratory pebble mill. An ore ground in fresh water in the laboratory pebble mill may show a different flotation result from one ground in the presence of oil in the same mill, but the effect here is largely one of the dispersion of the oil through the pulp and it appears that if, following grinding in the presence of fresh water, the pulp is subjected to sufficient premixing with the oil to cause a dispersion of the oil as thorough as that in the case of grinding in the presence of the oil, the flotation result will be the same.
Accessory Tests
Any testing for flotation, upon the results of which a mill design is to be based, should be accompanied by tests as to the behavior of the ore in grinding machines, mechanical classifiers and settlers, and on the concentrate to determine the ease with which it may be sunk and the extent to which it can be dewatered by settling and thickening. No finished design on anything more than test-mill scale should be embarked upon until such tests have been carried out in actual machines. But for preliminary work much information will be afforded to an experienced engineer by the following tests:
Grinding
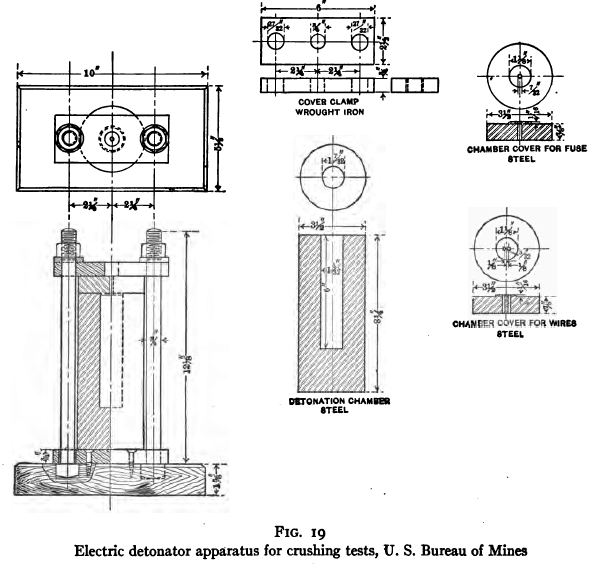
In a bomb of the dimensions and design illustrated in Fig. 19, imbed a No. 6 detonator with 100 gm. of a sand sized between 1.168 mm. and 0.833 mm., the sand having been obtained from a rock on which the performance of a given grinding mill is known. Explode the detonator, remove and size the sand. Run a duplicate test to insure the constancy of the exploding force of the detonator. Present-day detonators in good condition will give results that check within the limits of error of a screen test, i.e., the curves representing the sizing analyses will be practically coincident. Make duplicate tests in the same way on the ore under investigation. If the results of the duplicates again check, the force exerted by the four detonators may be concluded to have been constant and the results may then be compared directly.
Classification & Settling
Classifying and settling performances of an ore may be predicted from a comparison of the performance of samples with the behavior of like samples of a known ore whose mill performance is known. Thus artificial samples of the known and unknown ore made up to the same screen analysis should be stirred up in a beaker and the beaker then be allowed to stand for, say, one minute, at the end of which time the material in suspension should be poured into another beaker where it is again allowed to settle for a minute or longer as desired and the suspended solids again poured off. This manipulation should be repeated until enough points on a settling-rate curve are established so that this curve for the known and unknown ores can be compared. Knowing then the performance of given apparatus on the known ore, the performance of the same apparatus on the unknown ore can be predicted.
Concentrate handling consists in breaking down froth concentrate, thickening the same by settling, and filtering the thickened product. Sampling and transporting from thickener to smelter also offer problems, but these are not part of the subject matter under present discussion. A few general principles are all that can be set forth here to aid the experimenter
Froth may be broken down by impact or by the force of surface tension or both. Unfortunately the same forces also tend to make froth. Hence it is essential that they be utilized in a different way or to a different extent when the end in view is froth destruction. If a small amount of froth is placed on a body of fresh water or water but slightly contaminated with a frothing agent, the tension of the water surface will pull the froth mass apart into individual bubbles and will then so extend most of the individual bubbles, especially the larger ones, that the films will rupture and the solid load will sink. The bubble film may also be ruptured by piercing or puncturing. In practice this is accomplished by directing a spray of water upon the froth. A froth is a system in more or less unstable dynamic equilibrium under the forces of gravity, surface tension and viscosity. Anything that tends suddenly to upset the equilibrium of the system will tend to break down the froth. A sudden change in surface tension can be brought about by spraying with a substance or solution whose surface tension is different from that of the bubble films.
The three phenomena outlined in the last paragraph are all utilized in froth breaking. General practice in the mills is to run the froth concentrate through launders to Dorr tanks fitted with a peripheral curb to prevent froth overflow, and to spray the surface of the tanks, particularly near the center, usually with fresh water, in order to puncture the bubble films. Occasionally the water used is contaminated with a substance which markedly lowers the surface tension.
This upsets the equilibrium of the forces acting in the bubble films, in addition to the puncturing effect. This latter procedure is necessary only in the case of obstinately persistent froths.
Froths carrying a high percentage of solids are more persistent than those with a low percentage and more elaborate froth breaking equipment is necessary for their treatment. Such froths result from ores carrying a high percentage of mineral or high percentages of kaolinized matter. They result also from agitation methods of froth formation as differentiated from pneumatic methods.
Certain flotation agents, notably petroleum products and wood-tar oils produce persistent froths. Also the froths produced with more than 1 per cent, of oil on the ore are harder to break down than those produced with smaller quantities.
In-House Pilot Plant Testing
Pilot machines should form a part of every mill installation, especially in its early life. The pilot machine should give a quick and easily visible indication of the character of the plant tailing. Either a gravity concentrator or a pneumatic flotation machine may be used. In either case a portion of the general tailing of the flotation plant is diverted to the pilot machine and there treated slowly in order that all of the recoverable mineral may be exposed for inspection.
Probably the best pilot installation is a combination of the two machines, in which the gravity concentrator will show the coarse mineral that is being lost and thus guide grinding operations, while the pneumatic flotation machine will bring to view the finer mineral in the tailing.
https://www.911metallurgist.com/testing-flotation-oils-frothers
P. 111-125
