Listed and explained in details below are 8 Gold Assay Methods which are alternatives to the classic fire assay method.
Amalgamation Assay
This is useful in determining the amount of gold present in the ore capable of being extracted by mercury. The sample of ore is pulverised fine enough to pass through a 60- or 80-mesh sieve, made into a paste with a little water, an equal weight of mercury added, and the whole ground in an iron mortar with a pestle for from two to four hours, small additional amounts of water or mercury being added from time to time according to the appearance of the triturated mass. The consistency of the mass must be such that the globules of mercury do not sink in it but are broken up into very small particles. A little sodium amalgam dissolved in the mercury prevents it from flouring. The grinding is continued until the particles of ore are all impalpably fine. A machine for the purpose, called the “arrastra” mortar, has a large pear-shaped muller loosely fitting the inside of the mortar, and capable of being revolved in it by means of a handle. Complete amalgamation is performed in this machine much more rapidly than in an ordinary mortar. When the operator judges that amalgamation is complete, enough water is added to reduce the mass to a thin pulp and stirring is continued for a few minutes to collect the mercury at the bottom. The contents of the mortar are then “ washed down” in a pan, the mercury collected and distilled; and the residue, consisting of gold, silver and base metals, scorified with test-lead, cupelled, and parted. If it is not necessary to estimate the silver extraction, the mercury may be dissolved by nitric acid, and the gold cupelled and weighed.
The sample may of course be panned before amalgamation. In that case Charleton recommends treatment in a weighed golden dish, the surface of which has been amalgamated.
Gold Assay by Chlorination
Plattner’s method for the assay of roasted pyrites consists in placing the mineral moistened with water in a glass cylinder, 200-250 millimetres deep and 20-30 millimetres in diameter, and introducing a current of chlorine gas at the bottom. When the odour of chlorine is noticed above the ore, a cover is put on, the stream of chlorine stopped and the whole left for twenty-four hours, after which the reaction is complete if chlorine is still in excess. Boiling water is now run through the ore until all soluble salts have been washed out, and the gold contained in the solution is precipitated by ferrous sulphate, collected, cupelled, and parted. This method fails if more than about 20 per cent, of silver is present, as the chloride of silver formed encrusts the gold and protects it from the action of the chlorine. Balling recommends the addition of common salt to dissolve the chloride of silver, but found that the telluride ores of Nagyag yielded only 85 per cent, of their silver and 92 per cent, of their gold when successively treated with chlorine and sodium chloride.
Another method is to place the completely roasted sample in a stoppered bottle with enough water to make the whole of the consistency of thin mud. The ore and water should together occupy about two-thirds of the bottle. Bleaching powder and a thin glass bulb filled with dilute sulphuric acid are then added and the bottle securely closed. As cork is attacked by chlorine, glass or vulcanite stoppers are better, and the screw-stoppered soda water bottles are most convenient; if corks are used they must be wired down. The bottle is then shaken so as to break the sulphuric acid bulb and mix its contents with the bleaching powder, when chlorine is evolved. The bottle is now left for several hours in a warm place, being shaken occasionally by hand to mix its contents. At the end of a period of eight to twelve hours the bottle is opened, and if excess of chlorine is still present the liquid is separated from the ore and the latter washed thoroughly by filtration or decantation. The liquid and washings, whether clear or muddy, are warmed to expel free chlorine, and an excess of ferrous sulphate is then added to them. The precipitate is collected, scorified with lead, and cupelled. In all cases it is better to keep the first liquid separate from the washings, which should be concentrated by evaporation, since, if this is not done, the precipitate of gold may be too fine to settle and will pass through filter-paper. Bromine may be used instead of the materials generating chlorine. The quantities of chemicals required will be such as are sufficient to generate a volume of chlorine equal to twice the capacity of the bottle used, or to make a solution of 2 per cent, of bromine in water. Only finely- divided gold comparatively free from silver is extracted by this method.
Assay Metallic Copper and Copper Matte for Gold & Silver
Two methods are in use—a furnace assay method and a mixed wet and dry assay method.
Furnace Method
Ten scorifications are made each of 0.1 A.T. of the sample, 50 grammes test lead (using half as cover) and 1 gramme borax glass as cover. The lead buttons are cupelled separately and the beads weighed together, and parted. The cupels are refused in five lots of two each with 90 grammes of litharge, 50 grammes each of soda and borax, and 3 grammes argol. The results are higher than those obtained by the mixed method.
Mixed Wet and Dry Method
Weigh out 1 A.T. of the auriferous material, place it in a beaker of a litre capacity, and add gradually enough nitric acid of specific gravity 1.14 to dissolve it completely; heat until red fumes cease to come off, dilute to 600 c.c. with water, and add 10 c.c. of a concentrated solution of lead acetate and 5 c.c. of concentrated sulphuric acid, and allow the lead sulphate to settle. The precipitate is filtered off and washed. It contains the gold which has been collected and carried down by the sulphate of lead. The filter-paper and precipitate are dried, the paper burned, and the ash and lead sulphate scorified with test-lead. The button is cupelled and the gold with any trace of silver it may contain is weighed and then parted.
The silver is determined on a separate sample, which is dissolved and treated as before, except that a slight excess of sodium chloride, or, better still, of sodium bromide (Whitehead ) is added after the sulphuric acid and before the lead acetate. The solution is well stirred, and the precipitate allowed to settle and treated as before. The use of the lead acetate is to cause the precipitates of gold and silver to settle quickly, and to enable them to be filtered effectively. Sodium bromide is used instead of the chloride, on account of the greater insolubility of the silver salt.
Gold Assay of Purple of Cassius
One part of purple of Cassius is fused with three parts of carbonate of soda, cooled and dissolved in water. The gold remains undissolved, and is collected on a filter and cupelled after incineration.
Assay of Graphite Crucibles Method
Graphite crucibles, stirrers contain considerable quantities of gold and silver after use in melting bullion. They may be crushed with water by edge-runners in mills resembling mortar mills or Chilian mills (q.v.), and the products washed down to separate shots of metal. The residues, even after roasting, yield only a moderate proportion of their values when treated by amalgamation, and can still be profitably smelted. The sampling of the crushed material before amalgamation is difficult, and must be carried out with great care. The dried sample is roasted and scorified, or fused in pots at the Royal Mint, but Dr. Loewy obtains correct results by scorifying without previous roasting. He takes 5 grammes of the material, 60 grammes of lead, and a little borax or glass powder. The charge at the Royal Mint is—
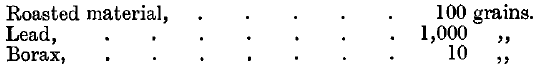
Half the lead is used as a cover. The slag is cleaned in the usual way, and is subsequently remelted in a crucible and a mixture of litharge and charcoal thrown on to the surface of the fused material. In this way from 0.5 to 2.5 per cent, of the total values are recovered from the slag. The cupellation loss is usually from 0.5 to 1 per cent.
Calculating Gold in Dilute Solution
A. Carnot has shown that the rose colour produced in presence of arsenate of iron is very sensitive, and can be used for colorimetric estimation of small quantities of gold. To attain this end a neutral solution of chloride of gold of known strength is prepared, and some drops of solution of arsenic acid added slowly to it. Then after a time two or three drops of dilute ferric chloride and some hydrochloric acid are added. If the liquid is not acidulated, a flocculent purple precipitate forms; if it is too acid the reaction fails, and only a faint blue colour is seen. The liquid is made up to 100 c.c. with distilled water, a pinch of zinc dust added, and the mixture shaken in a flask. A colour is produced, varying from rose to purple, according to the amount of gold present. The solution is clear, can be filtered unchanged, and kept without alteration for some time. If more than one milligramme of gold is present (1 in 100,000), the colour becomes too intense for small differences to be noticeable; if less than one-tenth of this amount is present (1 in 1,000,000), the colour becomes too faint. Between these proportions the amount of gold present in a liquid can be determined by comparison with a series of prepared coloured solutions.
For more dilute solutions of chloride of gold, the test described here, depending on the use of stannous chloride, may be used. A number of precipitates are prepared from solutions of gold containing known amounts, and compared with that given by the solution to be estimated. Suitable volumes of liquid from which the test precipitates are obtained are as follows:
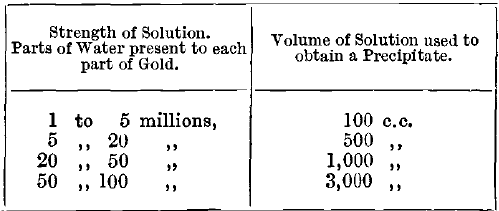
If more gold is present than one part per million, the colour of the precipitate is too intense for accurate measurement. Vanino and Seeman recommend the reduction of gold chloride by hydrogen peroxide in the presence of caustic soda or potash. The reaction is complete in a few minutes in the cold. In dilute solutions, the hydrogen peroxide is destroyed by heating the solution, and hydrochloric acid is added to agglomerate the precipitate. A solution containing 3 parts of gold in a million gives a slight red colour with a bluish shimmer.
The methods of estimation of gold in dilute solutions of cyanide are given on pp. 346 and 370.
Gold Assay by Electrolysis Method
If the gold is in solution, potassium cyanide is added in the proportion of 2.5 grammes per 100 c.c. of solution, and a current is passed through the solution between platinum electrodes. The current density should be about 0.3 ampere per square decimetre, the fall of potential 2.7 to 4 volts, and the temperature 50° to 60° C. The gold is deposited in about 1½ hours, and is weighed together with the cathode in the usual way.
According to F. M. Perkin and W. C. Prebble it is better to use a solution of ammonium thiocyanate instead of potassium cyanide. With a current of 0.4 to 0.5 ampere per square decimetre, deposition is complete in 1½ to 2 hours at ordinary temperatures. The best way of removing the deposited gold from platinum electrodes is by means of a solution of potassium cyanide containing an oxidising agent such as hydrogen peroxide, sodium peroxide, or an alkali persulphate.
