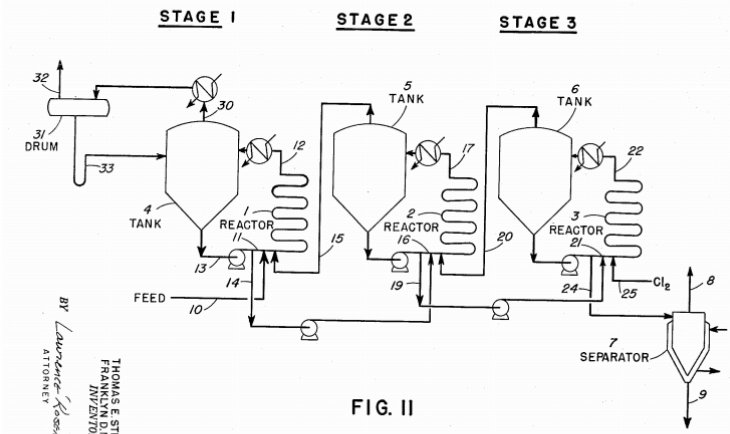
Miller’s Gold Chlorination process was introduced by F.B. Miller. The refining process employs chlorine gas, which passed into molten gold covered with a layer of borax and silica, and reacts with most of metals present in the molten charge. Platinum group metals do not react. Basically, gold is slightly attacked in the first moments and all the chlorides formed rise to the surface and are removed with the layer of borax and silica until the viscosity of the layer is sufficient to remove them. During the process some chlorides are volatilized and pass out of the furnace. Once is complete the initial reaction, there is a fast generation of non-volatile compound called Miller Salt that must be removed from the surface of the molten charge. It is important to avoid the formation of gold chlorides in order to avoid losses. One solution is to the problem is to cover the molten charge with mixture composed by borax and silica. When the metallic gold is as fine as 99%, gold chloride is produced in the molten charge in high levels and starts to appear in high proportion in the fumes that must contact the slag. Essentially, the addition of chlorine gas is reduced step by step. The indication of the end of the process is given by a peculiar colour on a cold pipe held in the issuing fume. At this moment, the addition of chlorine is stopped and the final slag is removed and the gold is poured in a cast. The final purity of gold is 99.5 to 99.8% and the minor elements are silver and small quantities of copper.
When the metallic gold is as fine as 99%, gold chloride is produced in the molten charge in high levels and starts to appear in high proportion in the fumes that must contact the slag. Essentially, the addition of chlorine gas is reduced step by step. The indication of the end of the process is given by a peculiar colour on a cold pipe held in the issuing fume. At this moment, the addition of chlorine is stopped and the final slag is removed and the gold is poured in a cast. The final purity of gold is 99.5 to 99.8% and the minor elements are silver and small quantities of copper.
https://www.911metallurgist.com/millers-gold-refining-process-equipment-supplies
It has been noted that the slag may content 1-2% of gold as small beads and tiny particles of gold produced by the reaction of chlorine gas and the molten charge. Usually, the gold is recovered by adding sodium carbonate to the molten charge. The best point of addition is over the slag, but the final decision depends on the experience of the operator. During the process, part of the silver is reduced due to the reaction between silver chloride and sodium carbonate, and the metallic product goes to the bottom. The product is retreated again and the silver trapped into the slag is treated separately. In order to optimize the process, some variations were introduced initially, but their results were not satisfactory. One of these changes was to add air after chlorine in order to oxidized part of the base metals.
With E.B. Miller’s Gold Chlorination Process of refining impure gold with chlorine gas (patented in Britain in 1867)
https://docs.google.com/viewer?url=patentimages.storage.googleapis.com/pdfs/US2997508.pdf