Though the gold recovery methods previously discussed usually catch the coarser particles of sulphides in the ore and thus indirectly recover some of the gold associated with these and other heavy minerals, they are not primarily designed for sulphide recovery. Where a high sulphide recovery is demanded, flotation methods are now in general use, but in the days before flotation was known, a large part of the world’s gold was recovered by concentrating the gold-bearing sulphides on tables and smelting or regrinding and amalgamating the product. Though the modern trend is away from the use of tables, because flotation is so much more efficient.
GOLD FLOTATION
The flotation process, which is today so extensively used for the concentration of base-metal sulphide ores and is finding increased use in many other fields. In 1932 flotation plants began to be installed for the treatment of gold and silver ores as a substitute for or in conjunction with cyanidation.
The principles involved and the rather elaborate physicochemical theories advanced to account for the selective separations obtained are beyond the scope of this book. Suffice it to say that in general the sulphides are air-filmed and ufloated” to be removed as a froth from the surface of the pulp while the nonsulphide “gangue” remains in suspension, or “sinks,” as the expression is, for discharge from the side or end of the machine.
For more complete information reference is made to Taggart’s Hand book of Mineral Dressing, 1945; Gaudin’s Flotation and Principles of Mineral Dressing; I. W. Wark’s Principles of Flotation; and the numerous papers on the subject published by the A.I.M.E. and U.S. Bureau of Mines.
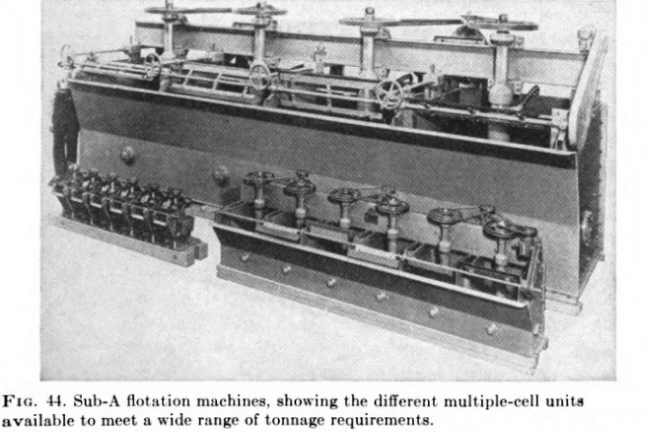
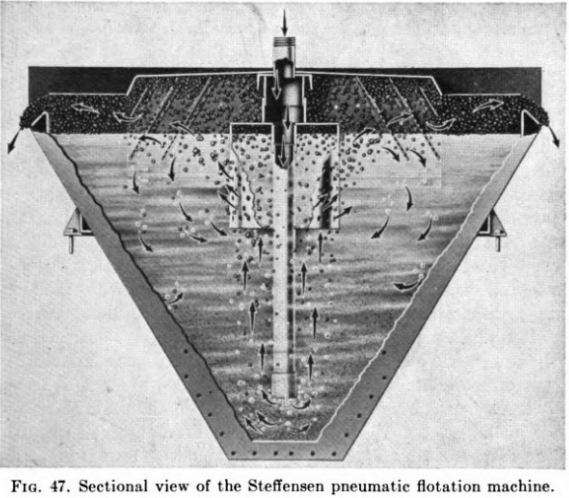
GOLD FLOTATION MACHINES
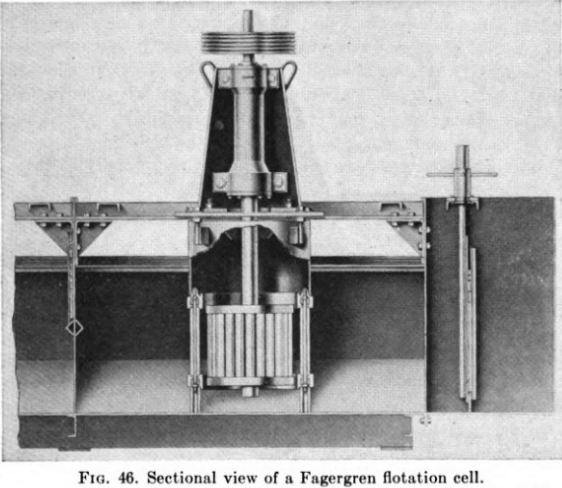
Flotation machines can be classed roughly into mechanical and pneumatic types. The first employ mechanically operated impellers or rotors for agitating and aerating the pulps, with or without a supplementary compressed-air supply. Best known of these are the Mineral Separation, the Fagergren, the Agitair, and the Massco-Fahrenwald.
Pneumatic cells use no mechanical agitation (except the Macintosh, now obsolete) and depend on compressed air to supply the bubble structure and to hold the pulp in suspension. Well-known makes include the Callow and MacIntosh (no longer manufactured) the Southwestern, and the Steffensen, the last, as shown in the cross-sectional view in Fig. 47, utilizing the air-lift principle, with the shearing of large bubbles as the air is forced from a central perforated bell through a series of diffuser plates.
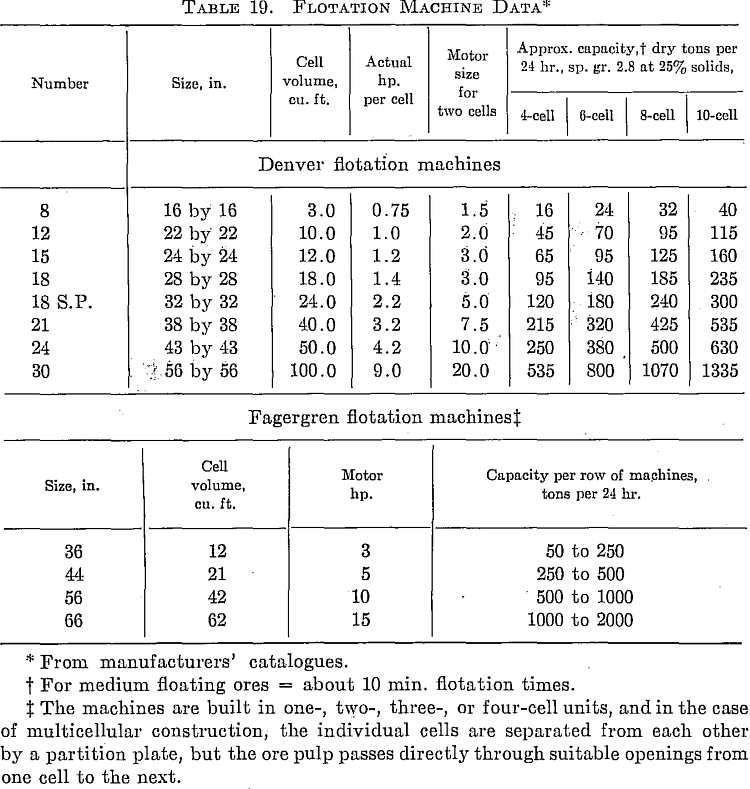
Calculating Flotation-cell Requirements
The number and size of flotation cells required for any given installation are readily determined if the problem is looked upon as a matter of retention time for a certain total volume of pulp. The pulp flow in cubic feet per minute is determined from the formula
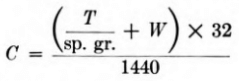
where C= cubic feet per minute
T = tons dry solids per 24 hr. in feed
sp.gr. = specific gravity of solids
W = tons of water per 24 hr. in feed
Having obtained the volume to be handled per minute, the total cell volume to be provided is found by multiplying this volume by the contact time in minutes required in the flotation machines.

For ordinary ratios of concentration the effect on cell capacity of concentrate (or froth) removal can be neglected, but where a high proportion of the feed is taken off as concentrates, or where middlings are removed for retreatment in a separate circuit, due allowance should be made for reduced flow and, in consequence, increased detention time toward the tail end of a string of cells. Not less than a series of four cells and preferably six or more cells should be used in any roughing section in order to prevent short-circuiting.
GOLD FLOTATION REAGENTS
It is not intended here to discuss the subject of flotation reagents in any detail. The subject is a large one with a comprehensive technical and patent literature. Research leading to the development of new reagents and to our understanding of the mechanism involved has been largely in the hands of academic institutions and the manufacturers of chemical products.
Recent work reported by A. M. Gaudin on the use of “Radioactive Tracers in Milling Research” described, for instance, the use of a flotation reagents containing radioactive carbon to determine the extent of collector adsorption. The “bubble machine” devised to measure the angle of contact of air bubbles on collector-treated mineral surfaces has been extensively used for determining the theoretical value of various reagents as flotation collectors, but for the most part the actual reagent combination in use in commercial plants is usually the result of trial-and-error methods.
The following is a brief discussion of the reagents ordinarily used for the flotation of gold and silver ores prepared from notes submitted by S. J. Swainson and N. Hedley of the American Cyanamid Company.
REAGENTS USED IN FLOTATION OF GOLD ORES
Conditioning agents are commonly used, especially when the ores are partly oxidized. Soda ash is the most widely used regulator of alkalinity. Lime should not be used because it is a depressor of free gold and inhibits pyrite flotation. Sodium sulphide is often helpful in the flotation of partly oxidized sulphides but must be used with caution because of its depressing action on free gold. Copper sulphate is frequently helpful in accelerating the flotation of pyrite and arsenopyrite. In rare instances sulphuric acid may be necessary, but the use of it is limited to ores containing no lime. “Ammo-phos,” a crude monoammonium phosphate, is sometimes used in the flotation of oxidized gold ores. It has the effect of flocculating iron oxide slime, thus improving the grade of concentrate. Sodium silicate, a dispersing agent, is also useful for overcoming gangue-slime interference.
Promoters or Collectors. The commonly used promoters or collectors are “Aerofloat” reagents and the xanthates. The most effective promoter of free gold is Aerofloat flotation reagent 208. When auriferous pyrite is present, this reagent and reagent 301 constitute the most effective promoter combination. The latter is a higher xanthate which is a strong and non-selective promoter of all sulphides. Amyl and butyl xanthates are also widely used. Ethyl xanthate is not so commonly used as the higher xanthates for this type of flotation.
The liquid flotation reagents such as Aerofloat 15, 25, and 31 are commonly used in conjunction with the xanthates. These reagents possess both promoter and frother properties. When malachite and azurite are present, reagent 425 is often a useful promoter. This reagent was developed especially for the flotation of oxidized copper ores.
The amount of these promoters varies considerably. If the ore is partly oxidized, it may be necessary to use as much as 0.30 to 0.40 lb. of promoter per ton of ore. In the case of clean ores, as little as 0.05 lb. may be enough. The promoter requirement of an average ore will usually approximate 0.20 lb.
Frothers and Froth Modifiers
The commonly used “frothers” are steam-distilled pine oil, cresylic acid, and higher alcohols. The third mentioned, known as duPont frothers, have recently come into use. They produce a somewhat more tender and evanescent froth than pine oil or cresylic acid; consequently they have less tendency to float gangue, particularly in circuits alkaline with lime. The duPont frothers are highly active frothing agents; therefore it is rarely necessary to use more than a few hundredths of a pound per ton of ore.
When coarse sulphides and moderately coarse gold (65 mesh) must be floated, “froth modifiers” such as Barrett Nos. 4 and 634, of hardwood creosote, are usually necessary. The function of these so-called froth modifiers is to give more stable froth having greater carrying power.
REAGENTS FOR SILVER ORE FLOTATION
The conditioning agents used for silver ores are the same as those for gold ores. Soda ash is a commonly used pH regulator. It aids the flotation of galena and silver sulphides. When the silver and lead minerals are in the oxidized state, sodium sulphide is helpful, but it should not be added until after the sulphide minerals have been floated, because sodium sulphide inhibits flotation of the silver sulphide minerals.
Aerofloat 25 and 31 are effective promoters for silver sulphides, sulphantimonites, and sulpharsenites, as well as for native silver. When galena is present, No. 31 is preferable to No. 25 because it is a more powerful galena promoter. Higher xanthates, such as American Cyanamid reagent 301 and amyl and butyl xanthates, are beneficial when pyrite must be recovered. When the ore contains oxidized lead minerals, such as angle-site and cerussite, sodium sulphide and one of the higher xanthates may be used. In some instances reagent 404 effects high recovery of these minerals without the use of a sulphidizing agent. Silver ores require the same frothers as gold ores—viz., pine oil, cresylic acid or duPont frothers.
“Aero,” “Ammo-phos,” and “Aerofloat” are registered trade-marks applied to products manufactured by this company.
The Great Western Electro-Chemical Company, California, makes amyl xanthate, butyl xanthate, potassium xanthate, and sodium xanthate. In the United States these reagents are used on the gold ores of California and Colorado and in Canada on the gold ores and sulphides of Ontario and Quebec.
Flotation reagents of the Naval Stores Division of the Hercules Powder Company are as follows: Yarmor F pine oil, a frother for floating simple and complex ores; Risor pine oil, for recovering sulphides by bulk flotation; Tarol a toughener of froth, generally used in small amount with Yarmor F, but with some semioxidized ores where high recovery is essential yet the grade of concentrate not so important, Tarol does good work; Tarol a frother for floating certain oxide minerals, but it can be used in selective flotation of sulphide minerals and in bulk flotation where tough froth is desirable; Solvenol, for the floating of graphite in conjunction with Yarmor F.
PRECIPITATING EFFECT OF FLOTATION REAGENTS
The statement has come to the attention of the American Cyanamid Company that organic flotation reagents, such as xanthates, even in the small amounts used in flotation, cause reprecipitation of gold from pregnant cyanide solutions. The ore-dressing laboratory of this company is studying the question, and preliminary results indicate that this statement is unfounded. The addition of xanthate, in the amount usually found in flotation circuits, does not precipitate gold from a pregnant cyanide solution containing the normal amount of cyanide and lime.
PRIMARY SLIME
Valueless slime, in addition to its detrimental effect in coating gold-bearing sulphide, thereby limiting or preventing its flotation, also becomes mixed with the flotation concentrate and lowers its value. Sometimes the problem in flotation is that, although the gold is floatable, the concentrate product is of too low grade. Talc, slate, clay, oxides of iron, and manganese or carbonaceous matter in ores early form slime in a mill, without fine crushing. Such “primary slime,” according to E. S. Leaver and J. A. Woolf of the U.S. Bureau of Mines, interferes with the proper selectivity of the associated minerals and causes “slime interference.” The tendency of primary slime is to float readily or to remain in suspension and be carried over into the concentrate. Preliminary removal and washing of this primary slime before fine crushing is one method of dealing with it. At the Idaho-Maryland mill, Grass Valley, Calif., starch is regularly used as a depressant during flotation. Flotation tests using starch were made on a quartz ore containing carbonaceous schist from the Argonaut mine, Jackson, Calif.; a talcose ore from the Idaho-Maryland mine mentioned; a talcose-clayey ore from Gold Range, Nev.; a siliceous, iron and manganese oxide ore from the Baboquivari district, Nevada; carbonaceous and aluminous slime from the Mother Lode and some synthetic ores. The conclusions from the foregoing tests were in part as follows:
- Finely divided metallic gold in milling ores floats readily, and a high-grade concentrate can be made by flotation if no interfering slime or gangue is present. Any good collector may be used for the flotation of gold, but organic collectors of the xanthate type produce a cleaner, higher grade concentrate than coal tar-cresote oils.
- Some “Protective Colloid” shoud be added to “wet out” talcose or carbonaceous slime and destroy its tendency to float.
- Clayey slime does not have strong flotative properties, but it tends to remain in suspension and coat mineral particles, making it difficult to obtain good selectivity during flotation. Proper deflocculation on an ore pulp and some agents to destroy the flotative property of clay improve flotation of this type of ore.
- It is essential to keep pulp containing iron (not hematite) and manganese oxides in a dispersed Condition, and improve results are obtained by means of a depressing agent such as starch.
- Starch was the most effective depressing agent tried. It should be added as a solution to the ore pulp. Starch displays a selective action in its depressing effect.
It acts first on the slime; then, if a sufficient excess of starch is present, it will cause some depression of sulphides and metallic gold, either by wetting out or by producing an extremely brittle froth. Therefore, care must be taken in regulating the amount of starch added to obtain the maximum depression of the slime commensurate with high recovery of the gold. In this, as in all other phases of flotation, each ore presents an individual problem and must be so studied.
It was describe by the use of 600 series of flotation reagents which were developed primarily for the purpose of depressing carbonaceous and siliceous slimes in the flotation of’ gold ores. Carbonaceous material not only greatly increases the bulk and moisture content of a flotation concentrate, but its presence makes cyanidation of the concentrate difficult or impossible owing to reprecipitation of the gold during treatment.
In the treatment of an auriferous sulphide ore associated with carbonaceous shale from South Africa, up to 77 per cent of the carbon was eliminated by the use of 1 lb. per ton of reagent 637 with a 90.5 per cent gold recovery at 20.4:1 ratio of concentration.
A gold carbonaceous sulphide ore from California carrying free gold yielded a 93 per cent recovery into a concentrate at 14.4:1 to ratio of concentration after conditioning with 0.50 lb. per ton of reagent 645.
In each case the ore was ground to about 70 per cent minus 200 mesh and conditioned at 22 per cent solids with the reagents as indicated. Flotation reagents included reagents 301 and 208 and pine oil. In the second case some soda ash and copper sulphate where also used.
GOLD FLOTATION FLOWSHEET
It is obvious that the most suitable treatment for ores carrying gold and silver associated with pyrite and other iron sulphides, arsenopyrite or stibnite, will depend on the type of association. Cyanidation is usually the most suitable process, but it often necessitates grinding ore to a fine size to release the gold and silver. Where it is possible to obtain a good recovery by flotation in a concentrate carrying most of the pyrite or other sulphides, it is often more economical to adopt this method, regrinding only the comparatively small bulk of concentrate prior to the leaching operation.
That the trend over the last 10 years has been in this direction will be noted from the numerous examples of such flow sheets in Canada and Australia (see Chap. XV). A number of plants formerly using all-cyanidation have converted to the combined process.
The suitability of the method involving fine grinding and flotation with treatment of the concentrate and rejection of the remainder should receive careful study in the laboratory and in a pilot plant. Mclntyre-Porcupine ran a 150-ton plant for a year before deciding to build its 2400-ton mill. Comparative figures given by J. J. Denny in E. and M. J., November, 1933, on the results obtained by the all-sliming, C.C.D. process formerly used and the later combination of flotation and concentrate treatment showed a saving of 12.1 cents per ton in treatment cost and a decrease of 15 cents per ton in the residue, a total of 27.1 cents per ton in favor of the new treatment.
Flotation may also prove to be the more economical process for the ore containing such minerals as stibnite, copper-bearing sulphides, tellurides, and others which require roasting before cyanidation, because this reduces the tonnage passing through the furnace.
Even when recovery of gold and silver from such ores by flotation is low, it may be advantageous still to float off the minerals that interfere with cyanidation, roasting, and leaching or possibly to smelt the concentrate for extraction of its precious metals. Cyanidation of the flotation tailing follows, this being simpler and cheaper because of prior removal of the cyanicides.
Flotation Unit in the Grinding Circuit
It is a good practice to recover as much of the gold and silver as possible in the grinding circuit by amalgamation, corduroy strakes, or other gravity means to prevent their accumulation in the classifier; otherwise gold that is too coarse to float may escape from the grinding section into the flotation circuit where it will pass into the tailing and be lost.
To prevent this, several companies including the Mclntyre-Porcupine at Timmins, Ontario, have inserted a combination of flotation cell and hydraulic cone in their tube-mill classifier circuits. At the Mclntyre- Porcupine, according to J. J. Denny in E. and M. J., November, 1933, this cell is a 500 Sub-A type. The total pulp discharged from each tube mill passes through 4-mesh screens which are attached to the end of the mills. The undersize goes to the flotation cell, and the oversize to the classifiers. Tailing from the cell flows to the classifiers, and the flotation concentrate joins the concentrate stream from .the main flotation circuit. The purpose of the hydraulic attachment is to remove gold that is too coarse to float, thus avoiding an accumulation in the tube-mill circuit. The cones have increased recovery from 60 to 75 per cent. Every 24 hr. the tube-mill discharge is diverted to the classifiers. Water is added for 15 min. to separate the gangue in the cells from the high-grade concentrate, after which a product consisting of sulphides and coarse gold is removed through a 4-in. plug valve equipped with a locking device. Each day approximately 400 lb. of material worth $2000 to $3000 is recovered. This is transferred to a tube mill in the cyanide circuit,with no evident increase in the value of the cyanide residue. The object of this arrangement is, of course, primarily to deplete the circulating load of an accumulation of free gold and heavy sulphides.
Flotation of Cyanide Residues
Flotation is used to recover residual gold-bearing sulphides and tellurides. The Lake Shore mill retreatment plant is an interesting example of this technique. The problem here was, of course, to overcome by chemical’ treatment the depressing action of the alkaline cyanide circuit on the sulphides. A full discussion of this and of the somewhat controversial subject as to whether flotation should in such an instance be carried out before, or after cyanidation will be found in J. E. Williamson’s paper “Roasting and Flotation Practice in the Lake Shore Mines Sulphide Treatment Plant” else where referred to. Summing up the specific considerations governing the choice of treatment, the author says:
CONCENTRATION
- Virtually all exposed gold and telluride values in the ore can be safely and cheaply extracted by cyanidation of the raw ore.
- The roasting of an uncyanided flotation concentrate made from the raw ore did not produce a product suitable for cyanidation.
- Cyanidation of a flotation concentrate made from the raw ore, though it would clean up the exposed values, was a difficult and costly treatment.
- The development of a cheap and practical method of conditioning the cyanide tails made it feasible to concentrate the values contained in the sulphides and treat them at a profit by roasting.
Incidental matters that influenced the choice of treatment scheme included the realization that preliminary flotation would have involved two separate treatment circuits with additional steps of thickening and filtration following the flotation. Furthermore, in the conditioning method evolved, as much as 60 per cent of the dissolved values in the cyanide tailings were precipitated and recovered.
There are, however, cases where flotation equipment was put in for the purpose of recovering the gold in a concentrate and rejecting the tailing only to find that the tailing was too valuable to waste and had finally to be cyanided before discarding.
It is generally true that cyanidation is capable of producing a tailing of lower gold content than flotation. At a price of $35 per ounce for gold this fact is of much greater importance than when gold was valued at $20.67 per ounce. The possible gold loss in the residue to be discarded will influence the choice of a method of treatment.
Cyanidation and concentration of gold and silver ores
