The bead of silver and gold obtained by cupellation is squeezed between pliers, or flattened by a hammer on a clean anvil, to loosen the bone ash adhering to its lower surface, and is then cleaned by a brush of wires or stiff bristles. It is then weighed, the silver removed by solution in nitric acid, and the weight of the residual gold taken, when the difference between the two weighings represents the silver. If the bead contains more than one-fourth its weight of gold, more silver is added to it as otherwise some of the silver will remain undissolved, being protected from the action of the acid by the outer layers of gold. The amount of silver to be added is calculated from the (approximately) known composition of the bead, or guessed from its colour. A pale yellow bead always contains more than 60 per cent, of gold, but a perfectly white bead may not “ part” completely. The addition of the silver is sometimes effected in the case of small beads by fusion on charcoal by the blowpipe, but it is better to cupel the bead with the additional silver, wrapped in as small a piece of lead foil as possible. This is called “inquartation.” The resulting bead is cleaned, flattened by a hammer, and dropped into nitric acid.
Even if the gold is less than one-fourth the weight of the bead, additional silver is sometimes required. When the amount of gold is small, it is convenient to use a large proportion of silver in parting. For example, if the gold weighs 0.2 mg., ten times its weight of silver answers very well. With smaller beards a greater proportion of silver is desirable. For 1 mg. gold, use 6 mg. silver. For 10 mg. gold, use 40 mg. silver. With these proportions, the gold does not break up if hot acid is used. If less silver is added, parting is not complete except after prolonged (20 to 30 minutes) boiling in acid.
A. Whitby considers f that for 1 mg. of gold about 4 mgs. of silver should be used, and for 0T mg, of gold about 12 mg. of silver. He states that the beads break up entirely or partially if larger amounts of silver are used. It is probable, however, that he is referring to parting which has been begun by dropping the beads into cold acid.
The parting is effected in a test tube or a porcelain crucible if the bead is small, in a “parting flask” if large. Nitric acid of specific gravity 1.25 (made by adding three parts water to four parts of ordinary strong acid) is suitable for use with all beads. There is no advantage in using more dilute acids for poorer alloys. The freedom of the nitric acid from chlorine is ensured by adding to it a little spent acid containing some nitrate of silver. In all cases the acids must be previously heated to boiling, as the gold does not in that case break up into such fine particles as if cold acid is used. The acid attacks the bead instantly and violently, turning it black and giving off nitrous fumes. Little beads containing only a small proportion of gold are dissolved in a few seconds, and decanting may be at once proceeded with. If the amount of gold is large, the boiling is continued for some minutes. Ten minutes boiling is enough for 10 mgs. gold and 40 mgs. silver. The acid is then poured off, the residue washed twice with boiling water by decantation, and if the bead is very large fresh acid is added of specific gravity 1.30 (two parts of strong nitric acid to one part of water). The boiling is then continued for five to ten minutes longer, when almost all the silver will have been dissolved. A very small amount of silver, weighing from 0.05 to 0.1 per cent, of the gold, obstinately resists the action of the acid, and remains as a surcharge which may be neglected in almost all ores. The gold usually remains as a single piece if it weighs less than 0.1 mg., even if the proportion is only one part of gold to 40 or 50 parts of silver. If the ore is rich, the gold sometimes, though rarely, breaks up if hot acid is used, and invariably breaks up if the parting is begun with cold acid. The finer particles may float and be lost in decantation. Particles floating on the surface may be sunk by touching with a glass rod or by a drop of water let fall on them. Continued heating of the gold in acid makes it agglomerate to some extent, so that it is easier to wash.
After the final decantation of the acid and washing twice with hot water, the water is drained off and the porcelain crucibles dried at a gentle heat, and then gradually raised to dull redness. The gold which was previously black and soft, being in a fine state of division, now resumes its usual yellow colour, and hardens so that it can be removed to the pan of a balance and weighed. At a bright red heat the glaze on the porcelain crucible softens and the gold sticks to it. Excessive heating must therefore be avoided. An ordinary Bunsen flame answers very well.
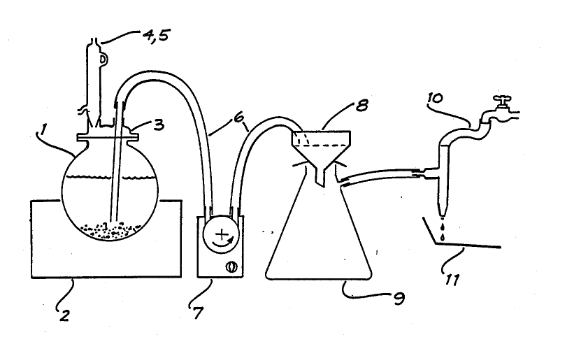
If a parting flask or test tube is used for the boiling, the parted gold is transferred to an unglazed Wedgwood crucible. To effect this the flask is filled with water, and the crucible placed over its mouth. On inverting both together the gold falls into the crucible, and the flask is removed in such a way as not to disturb the precious metal. The water which has filled the crucible is then poured off, and the crucible heated as before.
The chief difficulty in parting by the last-named method is encountered in transferring the gold from the glass vessel to the Wedgwood crucible; minute particles of gold may adhere to the glass and are then left behind. The loss of these is the cause of the low results occasionally observed when this method is used. If the glazed porcelain crucibles are used for both boiling and annealing, no transference from one vessel to another of the gold in its soft state is necessary, so that the source of error mentioned above is avoided. On the other band, the difficulty of boiling the acid in a small porcelain crucible without sustaining loss by projection may prevent the assayer from continuing the boiling for a sufficient length of time, and some silver may thus be left undissolved; in consequence of this the results obtained are sometimes too high by as much as 2 or 3 per cent, of the weight of the gold. Such errors would, however, be inappreciable in the assay of poor ores, and are the less serious for the reason that all the other sources of error (unclean slags, absorption by cupel) tend to make the result too low. The danger of loss by projection is avoided by using watch-glass covers for the crucibles. The latter are heated on clean asbestos sheets, not on sand baths. By assaying an accumulated stock of parted gold derived from ores, it was found at the Mint that the amount of silver retained by the gold when parted in crucibles as described above, is about 0.5 per cent.
To prevent “ bumping,” one or two small pieces of capillary glass tube or of clay pipe or other porous body are sometimes put into the acid together with the alloy to be parted, but, as a general rule, it may be assumed that parting is finished when bumping begins.
Whatever method of parting is used, very finely-divided gold may remain suspended in the liquids, and may thus be lost in the course of decantation. Loss by decantation after parting may be avoided by adding a globule of mercury free from gold, and stirring with a glass rod until all the black particles of gold have been absorbed. The spent acid is then poured off and fresh nitric acid of density 1.2 added. On gently warming, the mercury is slowly dissolved and the gold remains as a coherent spongy mass. It is washed with nitric acid and then with water, glowed, re-heated with concentrated nitric acid, washed, re-heated, and weighed.
By the operation of parting, silver, palladium, and some platinum are removed in solution, but part of the platinum, and all the rhodium, iridium, remain with the gold. If the presence of these metals is suspected they must be looked for and removed by special assay methods.
