In the case of many complex gold and silver ores roasting before cyanidation is essential if satisfactory extraction of the precious metals is to be obtained. In such cases no practical amount of grinding or prolonged contacting of the raw ore with cyanide solution will affect more than a certain low extraction; in other cases, while the extractions may be acceptable, the consumption of cyanide is prohibitive, and roasting or, occasionally, pyritic smelting of the material is the only alternative. Roasting, which is today almost always carried out following concentration of the values into a small bulk, must be used if it becomes necessary to decompose the minerals with which the gold is associated in order to expose it to solvent action. Many of the cyanicides in the raw ore are broken up and rendered harmless by roasting, but the calcine may contain new compounds that must be removed by water or acid washing before cyanide treatment can be successfully carried out.
There were instances, where at one time the whole of the ore was roasted before cyanidation, but this practice has been for the most part discontinued on account of the large plant and high overall costs involved. With improvements in flotation methods, the generally accepted procedure today is to concentrate the gold or silver bearing minerals into a small bulk and roast the concentrate only. The calcine is then usually treated in a small cyanide circuit, with the residues sometimes passing into the main cyanide circuit, if the flow sheet includes such a step, or sent directly to waste.
Those types of gold ores which most frequently require roasting include ores carrying arsenopyrite, stibnite, sulphotellurides, and pyrrhotite. Straight pyritic ores, where the pyrite is present in small quantities, usually yield their gold to fine grinding and cyanidation alone.
Silver ores containing the values as polybasite, stephanite, pyrargyrite (the antimony sulphide), and proustite (arsenic sulphide) usually require roasting. Tetrahedrite is often refractory even after roasting. Argentite (the silver sulphide) and cerargyrite (the chloride) can frequently be cyanided without roasting. A comprehensive description of roasting practice is to be found in a series of articles entitled “Roasting Gold-Silver Sulphide Ores and Concentrates” by M. W. von Bernewitz.
A more recent paper, “Roasting of Arsenical Gold Ores and Concentrates” by F. R. Archibald contains a bibliography listing 23 references on the subject of roasting gold and silver ores.
There are four general types of roasting furnace in use:
- the horizontal, multiple-spindle type, of which the Edwards furnace and modifications of it are typical;
- the rotary kiln;
- the vertical, multiple-hearth furnaces with rabble arms attached to a central shaft, of which the Wedge furnaces are typical; and
- the Dorrco FluoSolids reactor.
Recover Gold by Roasting Pyrite Ore
The roasting of straight pyritic ores involves the conversion of the iron sulphides to the oxide under oxidizing conditions with the evolution of sulphur dioxide and, to some extent by catalytic action, sulphur trioxide gas also. In this chemical change the iron mineral is rendered more or less porous, thereby permitting the dissolution of the contained gold by subsequent cyanidation.
Though simple in principle, considerable temperature and other controls are necessary to avoid undesirable side reactions which are discussed below. It is not usual, for instance, to leave more than about 0.1. to 0.15% insoluble sulphur in the roasted ore, but much larger percentages of soluble sulphates are often present. It is believed that in certain cases such salts may reduce the porosity of the iron oxide and so lower the gold extraction. In addition to higher gold losses, incomplete roasting also causes trouble due to the presence of ferrous salts and other cyanicides.
Roasting of flotation concentrates
The treatment scheme at Lake Shore includes direct cyanidation followed by flotation of the sulphides, roasting of the concentrates, and cyanidation of the calcine in a separate circuit. The following notes are taken from J. E. Williamson’s paper “Roasting and Flotation Practice in the Lake Shore Mines Sulphide Treatment Plant,” C.I.M. and M.
The pyrite values include all gold so intimately associated with the pyrite that cyanidation for a prolonged period under ideal laboratory technique will not dissolve it. Unlike the gangue values, finer grinding has comparatively little effect on reducing the pyrite values of the cyanide tailing.
Before the cyanide residue from the main plant can be floated, it is necessary to destroy the lime alkalinity present, and a good deal of experimental work was carried out before a suitable design of spray tower for conditioning the pulp with S02 gas from the roaster was worked out. This is fully described. Following recirculation of the pulp through this tower to give a pH of about 6.0 (see page 305), it is floated in Fagergren cells using reagent 301, copper sulphate, and pine oil. The concentrates are thickened, filtered on a drum filter provided with a flapper (see page 120), and fed by belt conveyor at 16 to 17 per cent moisture to the Edwards roaster. On the basis of a mill feed of 1200 tons per day, about 23 tons per day of concentrate carrying 26 to 27 per cent sulphur is roasted.

The installation includes two standard 70-rabble roasters, though only one unit is currently used. A 25-hp. motor drives the complete mechanism of each roaster. Special insulation was provided on the side walls and arch, and by using air-cooled rabbles a close control of temperature is possible without the use of any outside fuel.
A cooling hearth is incorporated in each of the Lake Shore roasters. This arrangement is made by providing a 10½-in. drop in the bottom of the roaster, which completely stops back mixing of the charge between the cooling hearth and the roaster proper. In the original roaster, eight pairs of rabbles out of the thirty-five are used for the cooling hearth. In the second roaster, the number was decreased to six pairs.
The wet concentrate is a sticky, puttylike substance which adheres to almost any dry surface.
The back mixing action of the rabbles ensures that there is always a quantity of dry, disintegrated charge at the feed end of the hearth. The fresh feed entering the furnace falls into this bed of dry, dusty material and picks up a coating of dust. This action can be compared with the baker’s use of flour to prevent bread dough sticking to his bread pans.
The dust-coated or dust-lubricated concentrate will not adhere to dry surfaces, nor will it ball up. The rapid turnover and thorough mixing given by the rabbles distribute the dust-lubricated material over the first three or four bays of the roaster, where it can be dried by the heat of the charge and the gases.
The first pair of rabbles are double armed to reduce the amount of wet feed which piles up under the chute between passes of the rabble. The moisture is driven off, and the filter cake shreds break up into more or less equidimensional lumps. On further application of heat, the trapped water inside these smaller lumps turns into steam. The pressure set up inside the lumps causes them to burst.
An over-all hearth slope of about ¼-in. to the foot is provided. However, it is stated that at no point in the furnace can the charge be said really to flow. During the elimination of the first atom of sulphur, indicated by the blue flame, the charge appears to be slightly lighter and fluffier. Even at this point, the angle of repose is over 30 deg. Elsewhere, the angle of repose is nearer 45 deg. These angles of repose compare with 35 deg, which is the angle for a rock dump.
This absence of flow makes it possible to work with a deeper bed of charge and enables the rabbles to do a more efficient job of turning the charge each revolution. The flocculent nature of the charge undoubtedly contributes materially to reducing the dusting and dust losses to the low figure obtained.
https://www.youtube.com/watch?v=glP9kUql2o4
The cooled calcine is discharged from the roaster by means of a classifier rake mechanism. The rake draws the calcine out well clear of the discharge port in the roaster before dropping it into the preliming agitator.
Operations at Lake Shore have demonstrated the vital importance of this temperature control over the initial stage of the roast. The charge must be held at a low temperature for a sufficiently long period during the blue-flame stage. (The temperature has reached 900°F. at the fifth port and rises slowly to a maximum of 1150° at the eighteenth port. Exit gases at 500 to 650°F. contain about 2.7 per cent S02.)
The ore is roasted to hematite rather than magnetite—the latter condition resulting in a higher cyanide consumption.
Roasting Arsenopyrite Ore for Gold
The roasting of ores containing arsenopyrite presents greater difficulties than straight pyritic ores mainly because of the tendency toward the formation of insoluble arsenites and arsenates, which have a detrimental effect on gold recovery.
In consideration of roasting procedure, it is quite generally agreed that reducing conditions should be maintained in the early stages of the roast to ensure elimination of the arsenic in the arsenious state. Provided this has been satisfactorily accomplished, the finishing stages of the roast can be done under active oxidizing conditions. It is not enough that reducing conditions alone be maintained during the period of arsenic elimination, as it is also necessary to maintain movement of the charge and a good flow of gas over or through the charge to carry off the arsenious oxide as it is produced.
A second important consideration in roasting procedure is that of the time-temperature sequence. For Beattie concentrate it proved best to hold the temperature about 900°F. during the period of arsenic elimination followed by a rise to 1300°F. before completion of the roast.
Roasting and leaching a sulphide concentrate
This 2000-ton per day plant is operating on an arsenical gold ore in the Duparquet area of Quebec, Canada. The operation consists of straight flotation, followed by roasting and cyaniding of the concentrates. The roasting plant, which is one of the finest installations in Canada, has paid for itself in increased recovery over that obtained by cyanidation of the concentrate direct.
The flotation concentrate is thickened, filtered to 16 per cent moisture, and partially dried (8 to 10 per cent moisture) in a coal-fired Buggies Cole drier. The discharge is in the form of round pellets, which are broken up by rollers placed on the inclined conveyors. It is delivered to 250-ton bins placed above each of three 25-ft.-diameter by 13 hearth Wedge split- draft roasters.
The furnaces are provided with an installation of two hot Cottrells for taking dust out of the hot gases, the 10 to 13 tons per day of dust carrying 2.25 per cent arsenic being returned to the third hearth by chain drags and elevators, and two cold Cottrells, which treat the gas after it has been cooled to 250° by addition of cold air (formerly by water sprays) to precipitate the arsenic.
There is also a powdered-coal-firing arrangement for adding heat to the roasters because of the low sulphur content (16 per cent) of the concentrate, and until recently heat was added to certain hearths continuously. At the present time supplemental heat is not supplied, and a feed carrying as little as 12 per cent sulphur plus 2 per cent arsenic has been successfully handled when a high tonnage rate (up to 190 tons per day) is maintained. The roasters have excellent insulation.
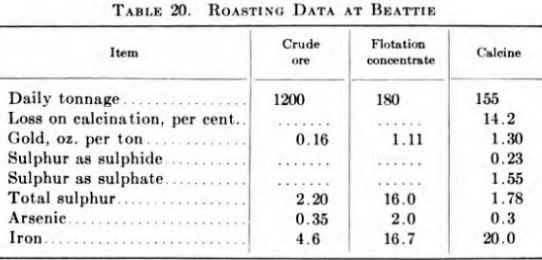
The roasters are operated on the split-draft principle; i.e., they are provided with bleeder flues from hearths 7 and 11 and two uptake flues from the first roasting hearths to a balloon flue. The As2O3, which condenses out of the gases when cooled below 500°F., is a product that is very corrosive and difficult to handle. The product from the Cottrells runs about 77 per cent As as AS2O3, 12 per cent SO3 and carries about 0.03 oz. Au per ton. As this is not high enough grade to meet present market demands, it is stored in concrete bins. The roaster-plant gases go to a 410 ft. high concrete stack, 18 ft. bottom and 7 ft. top diameter.
The calcine is quenched, without liming, and pumped to hydroseparators which are in closed circuit with tube mills, where the calcine is ground to about 90 per cent minus 325 mesh. This overflow is thickened, filtered, repulped in cyanide solution, and cyanided by a conventional flow sheet.
A total of 1066 tons of solution running about $5 per ton is precipitated per day. Zinc dust consumed equals 0.006 lb. per ton. The present over-all recovery is 85 per cent, consisting of a flotation recovery of 90 per cent, a roaster recovery of 99 per cent, and a cyanidation extraction of 93 per cent.
Table 20 summarizes the work in 1948.
The particle-size distribution of Beattie concentrate, as given by Archibald for 1940 practice, is shown in Table 21.
Conditions at various points in the roasters, in 1939 when the plant was operating full capacity are shown in Tables 22 and 23.
Roasting and Processing Sulpho-telluride ore to recover gold
This roasting plant, since closed down, was equipped with eight standard 70-spindle Edwards roasters, though only four were finally in use. Fuel was supplied to four ports on each furnace in the form of pulverized lignite of 8500 B.t.u. value prepared at a central plant. About 7 tons of fuel was consumed per 24 hr. for a rated furnace capacity of 120 tons of ore, which is equivalent to 117 lb. of coal per ton of ore roasted. The feed carried 3 to 4 per cent moisture, and the roasting temperature varied between 600 and 650°C. The time of contact was close to 5½ hr. The last 16 spindles operated in the open, and the air-cooled calcine after being sprayed with water was discharged by means of a reciprocating drag conveyor to a Cottrell precipitator. This unit resulted in improved extraction, since the fines were known to carry higher values than the ore as a whole.
Example:
The dewatered concentrates are passed over a Merrick weightometer into a 80-ton bin from which they are fed by a ribbon feeder on to the main roaster feed belt.
Eight Edwards duplex roasters are installed, and each is capable of handling 20 tons of concentrates per day. They are fed by a constant-weight feeder and are heat controlled by means of auxiliary off-take flues placed along the crown of the roaster. These flues draw off hot gases and reduce the temperature at any given point to the desired figure.
Ideal roasting is achieved when the temperature does not exceed 550°C. during the stage when pyrite is being oxidized to pyrrhotite, and this reaction is recognized by the lilac-colored sulphur flame.
The air is drawn through the roasters by two fans having a combined capacity of 100,000 cu. ft. per min.
The fans discharge to two six-cyclone Van Tongeren dust collectors and thence to a 9 ft. 6 in. diameter chimney stack 170 ft. high.
Loss of weight in roasting = 24.6 per cent.
FluoSolids Roasting System
Dorr-Oliver FluoSolids Reactor
Figure 49 shows a generalized cross-sectional view of the FluoSolids reactor which has recently been developed and is now being applied to the roasting of sulphide ores, to the calcination of various materials, and in general to problems involving reactions between solids and gases at elevated temperature.
In a recent paper on this subject delivered before the Canadian Institute of Mining and Metallurgy at the October meeting in Winnepeg, Manitoba, Owen Mathews states:
The term “fluidization” denotes the fundamental principle of carrying out a gas-solid reaction in a dense suspension of solids, maintained in a turbulent mass by the upward flow of the gases that effect the reaction. This mass assumes a fluid level and acts as a fluid. The upwardly moving gas stream imparts to this mass a turbulence resembling that of a boiling liquid.
The outstanding advantage of this principle, as embodied in the FluoSolids system, is the close degree of temperature control realized and the uniformity of the temperature condition that can readily be maintained throughout the fluidized bed. Under normal conditions there is no appreciable temperature difference between parts of the bed, nor is there any appreciable temperature difference between the gaseous phase and the solid phase.
This is believed to be highly important, because it means that the heat released is immediately distributed throughout the entire bed. Thus there is no observable local overheating at any point or points, resulting in fusion and locking-up of the gold values. Furthermore, heat losses are relatively low, leading to the belief that roasting can be properly carried out on sulfide concentrates containing as little as 12 per cent or even less sulphur and without the use of purchased fuel.
Roasting with a FluoSolids reactor
At Cochenour Willans, the FluoSolids reactor consists of a cylindrical shell built of ½-in. steel plate 18 ft. high with an inside shell diameter of 8 ft. 8 in., reduced to an effective inside diameter of 6 ft. 8 in. by a lining of 9-in. firebrick backed with 3 in. of insulating brick. The domed top of the reactor, the hot-air ducts, and the cyclone dust collectors are lined with a castable refractory cement. Appropriate openings are provided, piercing the steel shell and lining for pressure taps, thermocouples, and feed and discharge connections.
There are two outlets in the dome top of the reactor, one, 14 in. in diameter, being a gas discharge to the cyclone dust collectors and the other, 8 in. in diameter, being merely an auxiliary stack opening which is used only when the reactor is being preheated prior to being started up. Both are lined with refractory material. The main 14-in. gas line leads to two cyclone dust collectors arranged in series. The stack is 14 in. in diameter, built of 1/8-in. steel plate, and is 125 ft. high.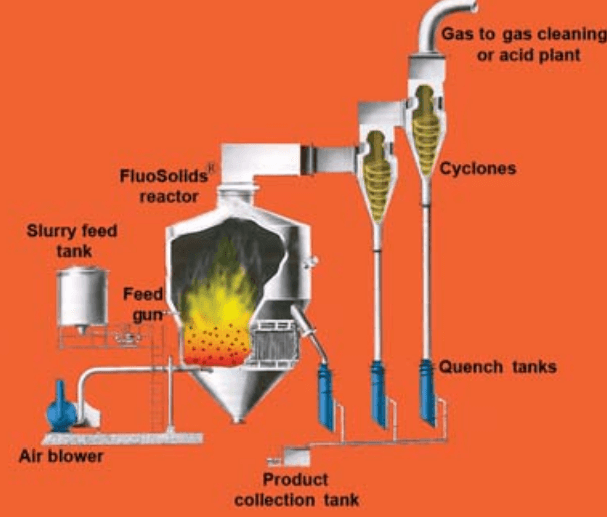
In the base of the reactor shell there is fitted a steel perforated constriction plate. This plate is lined with castable refractory cement and contains 120 cup-shaped orifices in each of which a 3-in. Korundal sphere is seated. These spheres act as distributing valves for the diffusion of the air throughout the bed and as check valves when the reactor is shut down or, in the case of power failure, to prevent calcine from passing down through the perforations into the conical air chamber below. Through the conical base and constriction plate an 8-in. pipe is fitted with an external butterfly for emptying the reactor when required. All external piping is of wrought iron.
Three quench tanks are provided to receive, respectively, the hot calcine from the reactor, the dust from the first cyclone, and the relatively finer dust from the second cyclone. All the quench tanks discharge to a common, screened receiving tank, the contents of which are pumped by a 2- in. rubber-lined sand pump to the cyanide plant.
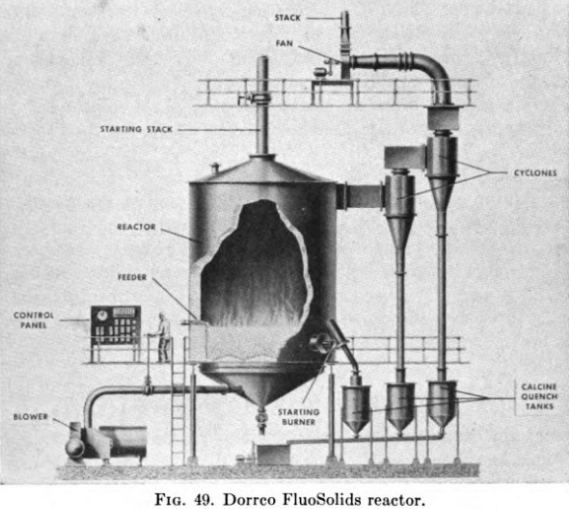
The arrangement of equipment directly ahead of the reactors is as follows: Flotation concentrates are pumped first to a small thickener then to a small rotary vacuum filter, with filter cake, at 12 per cent moisture or less, going to the reactor and with thickener overflow and filtrate going either to waste or back to the flotation circuit. The filter cake is fed to a 3-ton steel bin through a ¼-in. vibrating screen and is introduced into the reactor by a variable speed, Coghill-type feeder, consisting of a vibrating hopper and an 8-in.-diameter by-18-in. long ribbon-type screw conveyor, suitably designed to act also as a seal to prevent gas escaping from the reactor at this point.
The current tonnage of concentrates handled in the reactor is 8 tons per day assaying about 6 oz. gold per ton. Since the reactor has a rated capacity of 15 tons per 24 hr. (actually considerably more), it is operated on two shifts only. The reactor can be shut down and started up without difficulty and without any apparent disturbance in metallurgy or extraction.
Because the Cochenour Willans ore is self-roasting, temperatures are controlled at about 1100°F. by injecting water into the bed. Thermo¬couple connections at various points in the furnace permit automatic recording of temperature at about 5-min. intervals. Manometers connected above and below the constriction plate and at the top of the furnace indicate the pressure drop between various parts of the reactor.
For information on the cyanidation of the calcine see the general mill description in Chap. XV.
The Dorrco FluoSolids reactor is also available in multicompartment design, the top or feed compartment serving to preheat the ore and effect preliminary decompositions, such as dehydration or the elimination of arsenic, the middle compartment being the main reaction chamber, while the lower compartment serves to cool the calcine and preheat the incoming gases. The material passes down from one compartment to the next through “overflow” standpipes, and the countercurrent flow of gases and solids makes it possible to effect considerable heat economy where this is desirable.
Why use Salts in the roasting process
During the development stages of the roasting process at Lake Shore it was found that commercial cyanide extractions from the laboratory calcines could be obtained only from roasts in which salt had been added to the charge. This information was later confirmed in both the pilot plant and the commercial plant employing Edwards roasters.
The exact nature of the chemical reaction produced by salt, which has such beneficial effects in the cyanidation of the calcine, is not clear. While it can be demonstrated consistently on the Lake Shore concentrates, roasting tests carried out at Lake Shore on concentrates from mines in other camps failed to show any improvement when salt was added to the charge. These outside concentrates produced commercial cyanide extractions when roasted without salt.
The high degree of heat conservation in this type of furnace requires this step with ores of more than a certain critical calorific value in order to maintain the temperature at a predetermined figure.
Certain observations that were made on the effect of adding salt during the laboratory roasting test work have been borne out in the plant roasting.
- The cyanide extraction was raised from 70 to 80 per cent (obtained without salt) to 90 per cent when salt was added at the rate of 30 lb. per ton roasted.
- The presence of salt in the charge shortened the total time of roasting required to produce a dead calcine by from 10 to 20 per cent. This speed-up in the roast is accounted for by the charge commencing to calcine at an earlier stage in the roast and probably at a lower temperature than is the case when salt is omitted. This effect applied equally to those outside concentrates which could be roasted and cyanided successfully without salt.
- The calcine produced without salt from Lake Shore concentrates was a brown color. In roasts where salt had been added, the calcine was a rich purple-red shade.
A fourth effect was noted at the time of the laboratory roasting work, but the investigation of sizing technique has rendered this effect difficult to interpret. The calcine from salt roasts, when given the normal infrasizing treatment, appeared to be finer than the calcines produced with no salt.
The use of salt in roasting a gold-bearing concentrate requires a certain amount of caution. A process which depends on the use of excess NaCl and lime has been described for the extraction of gold from a concentrate by volatilization in a roaster. To avoid loss of gold, the amount of salt added must be held at such a figure that this volatilization does not occur.
The addition of 30 lb. NaCl to the Lake Shore concentrate does not cause any measurable volatilization of gold in the plant roasters.
ROASTING WITH SODA ASH
Studies by Queens University, Ontario, Canada, connected with special methods of roasting arsenical ores resulted in the finding that:
After heating arsenical ores or concentrates mixed with soda ash equal to 5 to 10 per cent of their weight in the absence of air for a period of 20 to 60 min. at temperatures between 950 and 1200°F. and then quenching in water, residues very low in gold values could be obtained by cyanidation of the calcine.
It was later found that 94 per cent of the gold in a 1.92-oz. concentrate could be extracted by quenching in water alone without the use of any cyanide at all.
It is known that gold forms soluble sulpharsenate and polysulphide compounds, and it is believed that the gold was taken into the quench solution through such agency. It could be precipitated from the solution by gentle aeration, particularly in the presence of a trace of manganese salt. Such precipitates assayed from 30 to 40 oz. per ton in gold, and the gold so precipitated was not readily soluble in cyanide solution.
There appeared at the time to be a number of difficulties attending the commercial adaptation of such a process, but as new roasting techniques are developed, it is probable that further work along these lines will be carried out.
GOLD LOSSES IN ROASTING
In general, serious gold loss by volatilization is not experienced in roasting gold ores or concentrates provided that the chloride content of the feed is below a certain critical figure (at Lake Shore this was 30 lb. per ton approximately).
Considerable gold loss may be experienced in the case of arsenopyrite ores, however, if the temperature is allowed to rise too rapidly during the fuming off of the arsenic. N. S. Spence of the Department of Metallurgy, Queens University, Kingston, Ontario, describes certain tests carried out in a laboratory muffle furnace as follows.
Samples for each run weighed 150 grams. A gas-fired muffle was used, which was brought up to the desired temperature before charging the roasting dishes. Every run was done in duplicate. The temperature was recorded every 5 min., and the average calculated. The temperature used was that of the atmosphere of the muffle directly above (¾ in.) the roasting dishes and was read from a thermocouple pyrometer. Roasting was continued in every test until no trace of SO2 could be detected by smell in the air above the dishes. This period of time averaged 95 min. During roasting, the charges were rabbled carefully every 5 min., great care being taken to avoid dust loss. Duplicate assays were run on the roasted product, and knowing the weight and the assay of each dish, the loss was calculated.
Calcination & Roasting Process
The usual method of process and treatment of the calcines (by calcination) from the roasting of gold ores is to cool in air, quench the moderately cooled material in water, and subject it to a light grind with pebbles or balls to break up agglomerated particles, sintered prills, etc. The ground pulp is then passed over corduroy or other types of blankets to trap any free gold released in the roasting operation, and then cyanided, by either continuous or batch system.
The above procedure is frequently modified to suit local conditions, both blanket, concentration and grinding being omitted in certain cases, while in others water and even acid washes followed by filtration before repulping in cyanide solution are resorted to.
A number of examples of calcine treatment will be found in Chap. XV, but the following may be considered typical of this operation.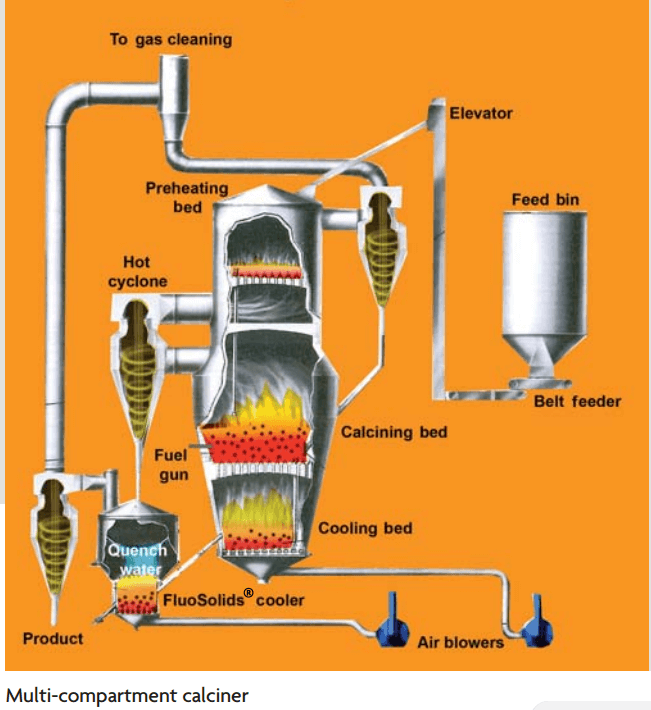
Preliming
As it leaves the roaster the calcine contains less than 0.1 per cent in soluble sulphur but as much as 2.6 to 3.0 per cent soluble sulphur (as sulphate) and a variable amount of chloride, which probably never exceeds 0.15 per cent.
The calcine has an acid reaction, and before it can be handled in contact with iron and steel, it must be neutralized. This is carried out in a preliming step in a 7- by 7-ft. turbo-agitator, which oxidizes the ferrous salts and precipitates the corrosive sulphates and chlorides present as hydrates. About 60 lb. lime per ton of calcine is used to bring the solution strength to 1.0 lb. CaO per ton.
Aeration
There are two stages of aeration in the treatment of the calcine pulp. The first stage is in the preliming agitator, where the pulp is given a thorough aeration. This aeration oxidizes the ferrous iron in the pulp to the ferric condition, in which form iron has a negligible cyanide consumption and no oxygen consumption. It also serves to saturate the pulp with oxygen before any cyanide is added.
The second stage of aeration is in the calcine agitation. At this point it is necessary only to maintain the oxygen content of the pulp. Aeration in the calcine agitators is obtained by blowing compressed air into the pulp through perforated pipes wrapped with six layers of canvas. These aerators are made of 8-in. lengths of 1-in. pipe, four per agitator. The aerators are located as near the bottom of the tank as possible to take full advantage of the increased solubility of oxygen in water due to pressure and to provide a maximum time of contact between the pulp and the rising column of bubbles.
The use of an efficient aerator in the calcine agitators has materially reduced the calcine cyanide consumption. However, the canvas sock aerator requires cleaning or replacement at 9-week intervals.
Cyanidation
It will be recalled that the flotation concentrate contains certain free gold values which resisted dissolution during the 60 hr. of main plant cyanidation. These values, after roasting, dissolve rapidly and completely.
The same rapid dissolution also applies to the values originally completely occluded in pyrite. The roast changes the pyrite grains into porous friable hematite which allows the cyanide solution to penetrate to and dissolve most of the gold values.
Test work has indicated quite clearly that cyanidation of the Lake Shore calcine is virtually complete after as short a treatment as 6 hr. After 9 hr. treatment, further time of contact will not lower the cyanide residue assay.
In plant-scale operations, the handling of a small tonnage of calcine presented certain problems. At a dilution of 2 to 1, a single 24- by 24-ft. agitator would, theoretically, furnish over 24 hr. agitation. However, short-circuiting would be so serious as to render any such treatment useless. It had been found in the main plant circuit that even six agitators in series did not completely overcome short-circuiting. However, a compromise was made in designing the calcine treatment plant. Four 12- by 8-ft. agitators were used in series. This provided some 24 to 36 hr. treatment. Thus, by more than trebling the time of contact known to be necessary for dissolution of the values, it was believed that the short-circuiting due to the use of only four tanks would be overcome.
The continuous calcine treatment required the use of a small Oliver filter. In view of the small tonnage to be handled, filtering costs per ton of calcine were high. The operation of the small filter was a constant source of inconvenience.
The high sulphate content of the calcine pulp gave rise to another problem. As the calcine pulp cooled during the agitation, CaSO precipitated. This formed a cement to bond together a heavy build-up of calcine around the rim of the agitators. The removal of this build-up necessitated shutting down the agitator and chipping off the accretions, which at times were well over a foot thick.
The continuous process was abandoned in favor of batch agitation. The calcine filter was scrapped, and batch filtration was carried out on one of the main plant filters.
The same agitators are used as in the continuous process, but for batch treatment they are arranged as two pairs of tanks. The pulp from the calcine mill is pumped to one of the pair of tanks until they are filled. The flow is then diverted to the other tanks.
Cyanide is added during the filling period so that, when the batch is filled, the cyanide is approximately at full strength. Additional cyanide is added as required to maintain a cyanide strength equivalent to 0.5 to 0.7 lb. KCN per ton of solution during the treatment.
The time of treatment, i.e., from the time the tanks are filled until the pulp is run off to the filters, varies from 12 to 20 hr. but is never less than 12 hr. This allows a sufficient margin of safety at all times.
One of the regular main plant filters is used to filter the calcine pulp. The main plant pulp is cut off shortly before the calcine batch is finished, and the filter is allowed to work out any solids remaining in its tank. Then the calcine pulp is fed to it.
Once batch agitation was instituted, most of the difficulty of a mechanical nature disappeared from the calcine cyanidation.
The danger of short-circuiting was removed. All the calcine pulp received a known time of treatment of sufficient length to ensure dissolution of all values recoverable by cyanidation.
The trouble previously experienced owing to build-up on the rim of the agitators disappeared. Since batch agitation, there has been no build-up at any point.
The precautions taken in cleaning out the residual main plant pulp before using a filter for calcine are necessary to prevent the calcine filter discharge from being diluted by the main plant pulp. It is desirable, for control purposes, to have a reliable assay of each batch of calcine residue.
Calcine pulps are considerably more difficult to filter than the normal main plant pulp. The spongy porous nature of the calcine adds to the difficulty of washing the cake. However, with careful operation it is possible to recover over 99.5 per cent of the dissolved values in a single stage of filtration.
For satisfactory filtration, the calcine cake must not exceed ¼ in. in thickness. Wash water must be used in sufficient quantity to cover the cake completely at all times. Failure to keep the cake covered results in the formation of cracks. Cracks in the filter cake provide a path of negligible resistance and so decrease the amount of wash water passing through the cake.
The filters are fitted with five spray pipes. The first three carry barren solution and the last pair fresh water.
The calcine treatment is carried out in a solution 0.5 to 0.7 lb. per ton of cyanide as KCN. Little improvement in gold extraction can be gained by the use of higher solution strengths, which do, however, result in higher cyanide consumption.
The roasted calcine and the furnaces
The roasted calcine withdrawn from the furnaces is collected in specially designed trucks. These act as cooling and storage bins, as well as a means of conveyance, and are fitted with a special bottom-discharge arrangement. By the use of these trucks the problems of cooling, storing, and dusting were eliminated, as no intermediate transfer of the calcine from truck to bin or bin to agitator was necessary. As each truck is filled with calcine, it is sampled and tested for unroasted calcine.
The hot properly roasted calcine is shunted onto a cooling track from which it is subsequently transferred to the leaching section of the plant.
About 330 gal. water and 200 lb. of 90 per cent sulphuric acid are added to the agitator, the paddle gear of which is then set in motion. Seven trucks of calcine, equivalent to 2.45 tons, are fed to the agitator, and agitation proceeds for 1 hr., after which the acidity of the solution is tested. The acid-treated pulp is fed by hose from the agitator to two 5- by 2-ft. box vacuum filters, each box taking about 22.5 lb. per sq. ft. of filter area.
A dry vacuum pump connected to the filtrate receiver is put into operation. Calcine pulp is fed to the filters to within 1 in. of the top of the boxes, and the vacuum valves are then opened to allow filtration to commence. When the original copper sulphate solution has been filtered from the calcine and the cake is surface dry, a water wash of 2 in. is run into the boxes, followed by five washes of 1 in. When full, the vacuum receiver is emptied via a 1-in. acid-resisting pump, which delivers the copper-bearing solution to two sand clarifier storage tanks, each 6 by 4 by 5 ft. 3 in. The clarified solution from these tanks is fed at controlled rates to two copper- precipitation boxes, wherein the copper is precipitated from solution on steel scrap. The barren solution is allowed to ran to waste.
The precipitated or cement copper is cleaned up twice a month and is one of the materials still dispatched overseas for realization, since all attempts to find a local market were unavailing, owing to the association of arsenic with the copper.
The cyaniding of the calcine is effected in three mechanical agitators, while the gold-bearing cyanide is separated from the calcine by means of a 3- by 2-ft. Denver rotary filter. The filtrate drawn from the Denver filter is pumped to two sand clarifiers, whence it gravitates to five filiform zinc-extractor boxes arranged in series. The extractor-box effluent gravitates to a storage sump, from which solution is drawn to supply the sprays on the Denver filter and for “make-up” solution required in the agitators.
The zinc-gold slime cleaned up from the extractor boxes is treated with sulphuric acid for the removal of the zinc and subsequently with nitro- sulphuric acid to remove any copper which has found its way into the cyanide section of the plant from the acid leaching section. The solution siphoned off after this operation is delivered to a “cyanide” cement precipitation box of three compartments, steel scrap again being the precipitant. The gold slime is then transferred from the acid vat to a filter box in which it is dewatered. The dewatered gold slime is calcined and subsequently fluxed as follows: gold slime (by weight) 58 per cent, borax 20 per cent, sand 12 per cent. The manganese dioxide is 10 per cent. The fluxed gold slime is smelted in a reverberatory furnace and poured into button molds. The buttons are then remelted in a carborundum crucible, granulated in water, and fluxed with 42 per cent (by weight) borax, 33 per cent sand, and 25 per cent sodium nitrate. This flux serves to remove bismuth which is present in the gold.
GOLD LOSSES IN CYANIDING CALCINES
An interesting problem confronting metallurgists is how to reduce the gold loss in the residues from the cyanidation of roasted calcines. An extensive investigation into this matter is reported in two recent papers published in Australia: “The Condition of Refractory Gold in Lake View and Star (Kalgoolie) Ore” by N. I. Haszard and “Roasting and Treatment of Auriferous Flotation Concentrates” by A. F. B. Norwood. The conclusion reached, as reported in the first of the above papers, was that of the three forms in which the gold occurs in Lake View and Star concentrates:
- as free gold,
- as gold tellurides,
- as gold associated with pyrite, the residue losses were almost entirely due to incomplete solution of the gold associated with the pyrite.
Apart from the evidence of microscopic study, roasting separately various sized fractions of the concentrate gave practically identical results, which again points to the extremely fine state of subdivision in which this refractory gold occurs. A series of tests also showed that the presence of calcium salts in the calcine had an adverse effect on cyanide extraction, presumably through the formation of compounds that reduce the porosity of the iron oxides resulting from the decomposition of the pyrite during roasting.
In the second paper, which reports a continuation of the same investigation, a rather detailed study was made of the chemical reactions taking place at various stages in the plant roaster by taking samples at various points along the hearth. An analytical method is described for determining elementary sulphur, sulphur as pyrite, and sulphur as pyrrhotite, also the proportions of magnetitie and hematite present in the calcine.
Tests were next carried out in which pyrite and pyrrhotite were roasted in sulphur dioxide. Since the reaction is endothermic, the temperature of the grains cannot rise above that of the furnace and the harmful effects of “flash roasting” is avoided. While the cyanide results were little better than the best roasting in air, it is worth noting that the magnetic or “black roast” is quite as amenable to cyanidation as the conventional “red roast.”
As a result of tests carried out at various temperatures, it was concluded that the high cyanidation tailings obtained following the higher temperature roasts are due to recrystallization of the iron oxide which destroys the porous structure induced by roasting and consequently locks up the submicroscopic particles of gold in dense crystals of hematite. Various methods for reducing the rate of combustion in plant furnaces are discussed.
Residual values of about 1.8 dwt. per ton appear to be the lower limit with present practice. While laboratory attempts to sulphate roast have not been very successful, the conversion of the calcine to ferric sulphate by strong sulphuric acid and subsequent ignition back to ferric oxide provides a material which will give almost 100 per cent extraction to cyanide. It is the opinion of the author that future improvements in recovery would appear to lie in the direction of devising a method of speeding up the oxidation of pyrites at temperatures below 500°C.
The gold can be removed from calcines by volatilization with salt in an oxidizing atmosphere, and also by smelting with lead, but there are economic and technical problems involved in both of these schemes.
Gold-silver-arsenic Alloys
Tests carried out by the staff of Nepheline Products, Ltd., in Canada in cooperation with F. P. Archibald to determine the solubility of gold-silver-arsenic alloys in the cyanide solution led to the following conclusion:
Arsenic, alloyed with gold-silver alloys, aids rather than hinders dissolution of gold in cyanide solution, and such could be ruled out as a cause of refractory behavior of gold-bearing arsenical ores. The presence of particles of precious metal in cyanide solution residues remains unexplained.
