The guard pot, with the clay pot in it containing 2 or 3 ozs. of fused borax, is placed in the furnace, and is heated gradually until the bottom of the clay pot is dull red. The ingots (of which the larger are slipper-shaped) to be refined, amounting in all to 650 to 720 ozs. in weight, are then placed loosely in the pot, the furnace filled with fuel, and the dampers opened. As soon as the gold is melted, which generally happens in about one and a-half hours, the boraxing of the pots being also affected at the same time, the perforated lid is put on, and the pipe-stem, previously brought carefully to a red heat to prevent cracking or flaking, is pushed to the bottom of the pot. As the pipe is being inserted, the chlorine is gently turned on to avoid stoppage of the passage through the stem by the solidification of metal in it. The supply of chlorine is controlled by the glass stopcock over the furnace, and the amount is adjusted so that the whole of the gas is absorbed and no globules of metal can be thrown up. This can usually be ascertained by feeling the pulsations of the gas through the indiarubber connections as it escapes in bubbles out of the bottom of the pipe-stem. When the gold contains much silver or base metals, the absorption of the chlorine takes place rapidly but gently, very little motion of the contents of the crucible being apparent, but when the gold to be refined is of high assay and also in all cases towards the end of refining, the gas is admitted only in a small stream, and requires careful watching to prevent spirting. When base metals are present in large quantities (over 2 per cent.) dense characteristic fumes of the chlorides of these are given off, and the metal or metals present may be generally identified by the fume or incrustation caused by the condensation of the base chlorides on the pipe or lid.
Gold containing 2 per cent, of silver and 0.5 per cent, of base metal is refined to about 995 fine in one and a-half hours, while that containing 3.5 per cent, of silver and 1.5 per cent, of base metals takes two hours. When larger percentages of silver or base metals are to be dealt with, the time taken is not proportionately longer, because, as mentioned above, a much greater stream of gas may with safety be admitted, though, in all cases, at the beginning the chlorine must be introduced gently on account of there being air in the chlorine mains, and, also, at the end of refining, the supply must be greatly reduced. When nearing completion, the “ flame ” issuing from the holes in the lid becomes altered in appearance, and much smaller; it now contains much chlorine mixed with small quantities of the volatile chlorides. The actual completion of the operation is generally known by the appearance of a very characteristic “ flame,” which is luminous, with a dark brown fringe. In case of doubt, a piece of clean pipe-stem is used as a test. It is placed, cold, for a few seconds in the issuing flame, and if the refining is finished, a clear reddish-brown stain, tending to yellow, is imparted to the test end. This stain consists of ferric oxide and chloride from the oxidation of ferrous chloride, and contains gold and sometimes chloride of silver, and is probably caused by small quantities of chloride of iron retained by the fused gold and non-volatile chlorides, from which it is freed by the unabsorbed chlorine bubbling through. Traces of copper and iron are always found in the refined gold, the bulk of the alloy being silver. As soon as the stain is found to be of the right colour, the current of gas is at once stopped, and the pipe is then withdrawn and the clay pot lifted out of the guard. If the current of gas is continued a loss of gold ensues. The pot is allowed to stand under a hood (to carry off the fumes) until the gold is set, which usually takes place in from five to seven minutes, the fact that solidification has taken place being observed by thrusting a piece of red-hot pipe-stem down through the fused chlorides. The chloride is then poured into a mould provided with a ventilating hood, which, in consequence of the high density of the fumes necessitating a sharp, draught to remove them, is connected with the stack. Any borax poured off with the chlorides is allowed to remain, as it is required as a cover for the chlorides in the subsequent fusion for the separation of their gold contents. The pot is then broken, as the cone of gold will not fall out of it soon enough, and the cone of refined gold is remelted in the guard and cast into two flat ingots, 12 inches by 4 inches by 1½ inches, which, when set and still red hot, are placed on a copper lift, dipped in dilute sulphuric acid and then in water, and after removal from the water are still sufficiently hot to dry by their own heat. The broken pots are ground in a small Chilian mill and panned off, and the gold obtained is added to the “ end ” that is returned at the end of the day. 9,000 ozs., containing up to 10 per cent, of silver and base metals, constitute a day’s refining.
An improvement has recently been introduced, by which a considerable saving of time and material is made. This is the dipping of the fused chlorides and borax from the pot while it is still in the fire, and without previous solidification of the gold, by a small clay crucible, from which they are poured into a covered mould projecting over the furnace, the drops falling back into the pot. This had previously been the practice when the percentage of silver was large, as the silver doubles its bulk on conversion to chloride, and would have overflowed. The last “dip ” always contains some gold, and is poured into a separate mould, in which the metal sets at once. The chloride is thence poured into the larger mould, and the gold returned to the pot. The chloride remaining in the pot is then made into a paste with bone ash, after which the refined gold is stirred and cast into ingots, the pot being at once returned to the furnace to be used a second time.
The chlorides, which hold from 5 to 10 per cent, of gold in feathery particles, are remelted during the day in plumbago crucibles holding 300 ozs. of chloride. When fused, 7 per cent, of their weight of bicarbonate of soda is added, cautiously and without stirring, which produces a shower of globules of reduced silver, and these falling through the chlorides carry down nearly all the gold. As one addition of bicarbonate of soda does not entirely free the chlorides from gold, a second addition is made, without removing the crucible from the furnace, ten minutes being allowed after each addition. The pot is then lifted out and placed on one side to allow the metal to set, when the chlorides are poured into a mould 12 inches by 10 inches by 2 inches, practically free from gold and ready for reduction. The silvery button obtained contains from 40 to 60 per cent, of gold, and is refined on the following day.
The silvery ingot and the refined gold contain 99.95 per cent, of the gold issued in the morning for refining, the bulk of the deficiency being in the pots. The amount of gold which goes immediately into work after refining is 97.6 per cent, (an average of thirty days’ refining). The amount of gold left in the silver after reduction from the chloride reaches a maximum of 1 part in 10,000, but is usually from ½ to 1/3 of this quantity.
The cakes of impure chloride, weighing about 250 ozs. each, may be colourless and translucent to brown or chocolate colour and opaque, the colour depending on the amount of copper salt present. They consist of argentic and cuprous chlorides, with traces of other chlorides and 9 per cent, of chloride of sodium from the decomposition of silver chloride by bicarbonate of soda. When cool, each cake is sewn up in a coarse flannel bag to prevent loss of any silver which may become detached during reduction, and they are then boiled with water in a wooden vat for four or five days. By exposure to air and moisture the cakes become coated with a green deposit, owing to the conversion of the cuprous chloride into cupric oxychloride and hydrated cupric chloride, and the successful removal of a large proportion of the cuprous salt in the vat is due to its solubility in a hot solution of common salt and of hydrated cupric chloride, from which it is redeposited on dilution.
The cakes for reduction are placed alternately with wrought- iron plates 1/8 inch thick in a cast-iron tank lined with similar plates. The plates are prevented from touching the bags by laths of wood, otherwise the copper would be reduced in the bags, and would be difficult to separate from the silver. The reduction is slow in starting, unless either some liquor is left from a previous operation or some chloride of iron added. The bath is heated by the direct injection of steam (this is absolutely necessary), and the reduction is complete in from two to four days, though sometimes it takes longer. The time may be lessened to twenty-four hours by putting the chloride cakes in metallic connection with the iron plates. The completion of the reduction may be easily ascertained by feeling the cake, when, if any chloride be unreduced, it is left as a hard lump. The reduced silver is taken out of the bags, washed in boiling water for about an hour, and then melted with from 1 to 5 per cent, of nitre. The use of the flannel bags makes the reduced silver of high standard, as the reduced copper is thus prevented from adhering to the silver cake, from which it was found very hard to detach it without tearing off silver as well. A small percentage of reduced silver and silver chloride is found at the bottom of the tank, its presence being probably due to the solubility of the chloride in solutions of the chlorides of copper, iron, and sodium.
The mean fineness of the refined silver was only about 930 prior to the year 1889. In 1889, after the introduction of the iron plates in place of zinc, the fineness rose to 948. The subsequent improvement to about 990 was due to the long continued boiling in water in flannel bags. When base gold is refined the amount of copper chloride is large and is probably more easily dissolved in water, so that the resulting silver is finer than if the original bullion contained only a small amount of copper. In the latter case the copper chloride is not easily removed from the chloride cakes.
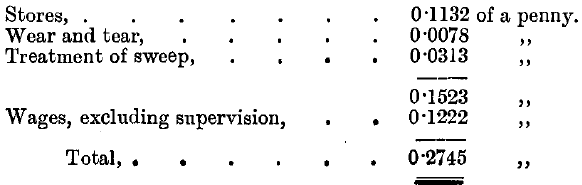
The silver contained in the gold operated on is distributed in the following manner, the mean results of the five years 1891-95 being given:

The “ sweep ” from the condensing chambers amounts to about 3 cwts. per annum, and contains an average of 41 ozs. fine gold and 157 ozs. fine silver, which are carried over as globules or volatilised as chloride and condensed.
The mean amount of gold refined per annum during five years (1891-95) was 949,527 ozs., containing gold 937.7, silver 49.6, and base metals (by difference) 12.7. The mean assay of the refined gold for the same period was 995.9, and the mean loss of gold in the refining operations for the same period was 0.175 per thousand. The approximate cost of refining per ounce gross weight refined was as follows:
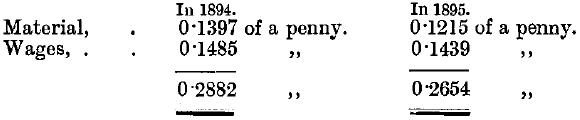
Half the cost for materials was for hydrochloric acid only.
In now billions of ounces of rough gold were chlorinated. The average assay before refining was:
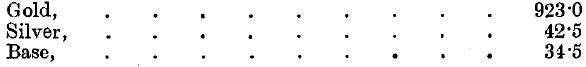
This was brought up to an average fineness of 994.9, costing approximately per gross ounce treated:
Edwards’ Generator was patented by T. Edwards, Pyrites Works, Ballarat, was introduced at the Melbourne Mint. The design is simple, and it has given entire satisfaction. It consists of a wrought-iron flanged cradle (see Fig. 89 which is from a working drawing kindly supplied by Mr. F. R. Power), fitted with a lead lining 5/8 inch thick, cast or hammered from sheet-lead to fit. The lining is supported at the bottom and sides by outside ribs (forming part of the casting), with openings to permit of the free circulation of the hot water used for heating the charge. The water passes between the iron case and the lining through a hole in one of the trunnions on which the generator is suspended, and escapes through a similar hole in the other trunnion. A flat lead-lined iron cover lies on the flange of the lining, and is secured by iron bolts to the flange of the cradle, a gas-tight joint being obtained by a rubber washer 3 inches wide.
There are four openings in the cover, three being 6 inches in diameter (though two of these only are absolutely necessary). These have lead flanges 1½ inches wide and lead-lined iron covers, which are kept in place by stout screws, passing through iron clamps bearing against iron lugs. One opening is used for charging-in and washing out, and the other has attached to its cover two pipes, one for water, which empties the generator of chlorine by forcing it through the second pipe into a second generator, when the charge of the first is exhausted.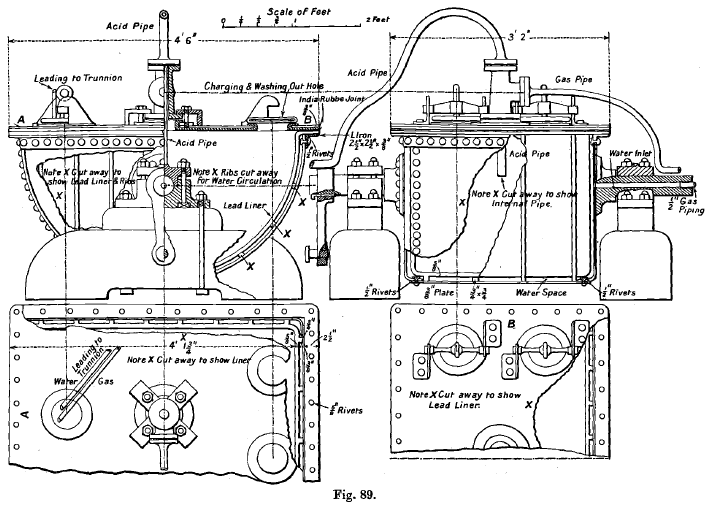
The fourth opening, 4 inches in diameter, in the centre of the cover, has a two-way lead casting with flanged openings, fastened over it. Through this the acid is delivered by a siphon pipe, while the chlorine passes through the second opening into the pipe leading to the condensing jar, and thence to the main pipe. The acid delivery, chlorine, and water pipes are bent over to a trunnion, and fastened to it by clips, and have 2 feet of stout rubber tubing, 3/8 inch wall, inserted in their length to take up the slight twist produced when the generator rocks. The acid is fed by gravity from a graduated lead jar, 7 feet above, by a lead siphon, having an earthenware tap at the level of the generator to regulate the flow.
A crank shaft connecting with the driving arm fixed at one end of the generator gives three complete oscillations per minute, the cover making an angle of 50° with the floor. This ensures the thorough mixing of the charge.
The usual charge is 80 lbs. manganese ore (73 per cent.), 100 lbs. salt, and 10 gallons of water. The acid is allowed to flow down at intervals until about 225 lbs. of chamber acid (sp. gr. 1.83) have been added. To get the maximum yield of chlorine, the charge must be heated nearly to boiling at the end. The generator is easily emptied through a 2-inch pipe burnt into one of the covers.
The capacity is about 90 gallons, and with 100 lbs. manganese ore, 125 lbs. salt, and 265 lbs. of acid, 1,560 ozs. of base metal and silver have been removed at the Melbourne Mint in one working day.
