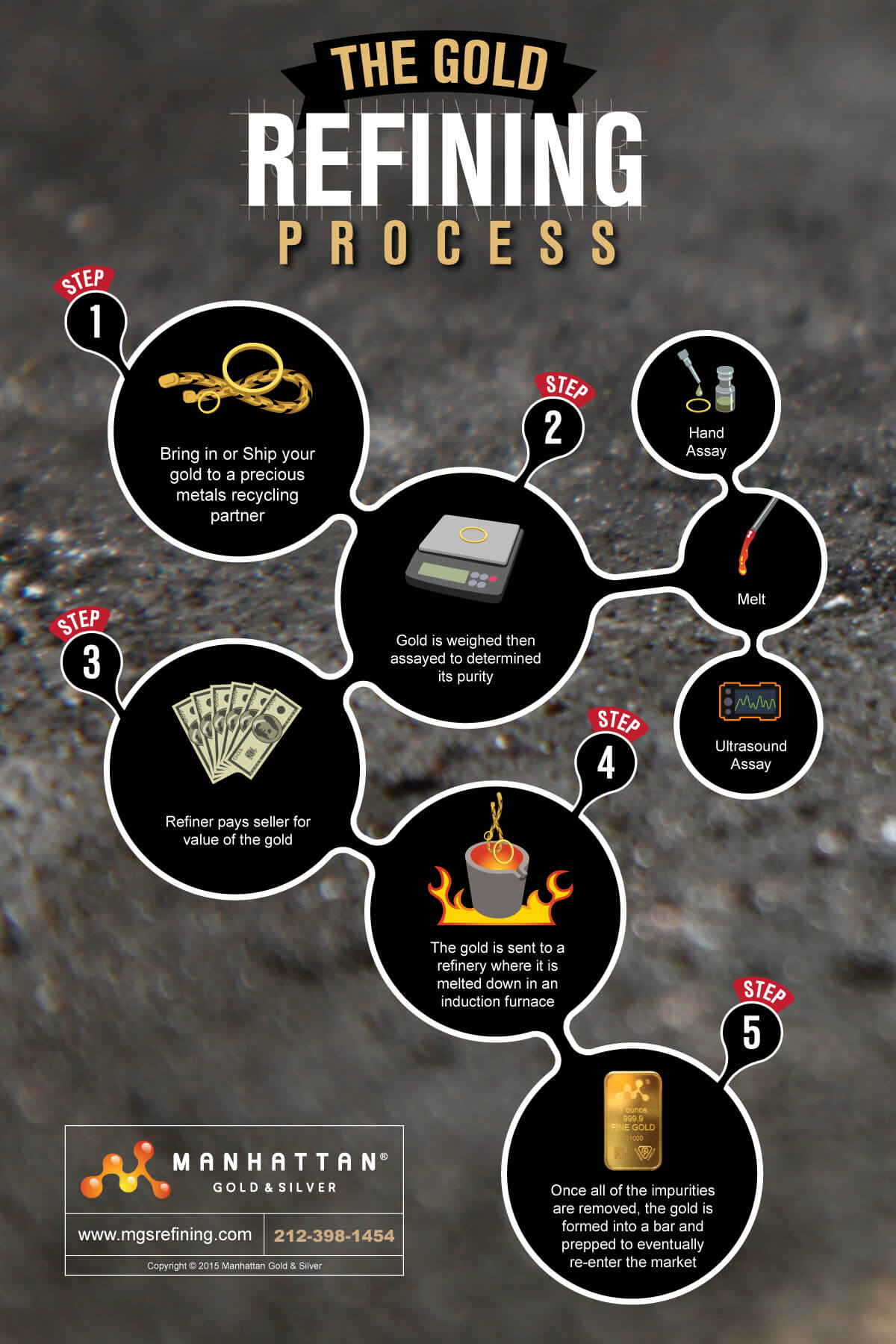
The processes used for Gold Refining are as follows:
- Volatilisation.
Oxidation
(a) by air blowing or roasting.
(b) by “ bessemerising”
(c) by nitre.
(d) by metallic oxides.
(e) by cupellation. - Chlorination.
- Sulphurisation.
- The use of iron or carbon.
The method to be used depends partly on the composition of the bullion, and partly on the means at the disposal of the operator. Cupellation belongs properly to the metallurgy of silver and lead, and need not be dealt with here. (See, however, Tavener’s process, p. 303). The other operations, except roasting, are usually carried out in crucibles, although reverberatory or tilting furnaces are sometimes used for work on a large scale.
Refining Gold by Volatilisation
The volatile metals are partly removed by melting the bullion. Mercury is almost all volatilised when the gold is melted. Mercury boils at 354°, sulphur at 440°, arsenic at 550°, selenium at 680°, cadmium at 770°, zinc at 940°, and tellurium at about 1,000°. Pure gold melts at 1,064°, but its melting point is lowered by the presence of impurities, and the temperature of molten bullion in an ordinary furnace may be taken as between 1,000° and 1,100°. At this temperature part of each of the elements mentioned above is volatilised, and the higher the temperature and the longer the time during which the metal is kept melted, the smaller will be the proportion of the volatile elements retained by the gold, but they cannot be entirely removed by heat alone. This is due to the formation of compounds with gold and silver, which are not readily decomposed by heat.
The losses of gold and silver by volatilisation are small. At 1,100° their vapour pressures are insignificant, and are independent of the presence of the impurities named. If, however, a large proportion of volatile elements are present, and the mixture boils, gold and silver are carried off mechanically as spray owing to the bursting of the bubbles, and the losses may be serious. The losses are increased by the passage of a current of air over the surface of the molten metal, and consequently it is better to cover the metal with charcoal, bone ash, fluxes, or a crucible lid. The use of dust-chambers is desirable.
The losses by volatilisation or as spray are higher in some of the other refining processes, so that dust-chambers are the more necessary when these are used.
Refining Gold by by Oxidation
Using Air to Refine Gold by Oxidizing
The use of a blast of air on the surface of molten gold or silver is probably the oldest of the refining processes. The method appears to be described in the Book of Ezekiel, to which the date B.C. 593 is assigned, and the principle is the same as in cupellation, which was described by Diodorus Siculus about B.C. 200. In cupellation, the oxides of the base metals are dissolved and slagged off by litharge, but if a blast of air is used when lead is not present in large quantities, it is necessary to add other fluxes as many oxides are almost infusible by themselves. In 1580, Ercker recommended the metallurgist to melt brittle gold “with good Venetian borax and drive it before the bellows till it endureth the blowing.” The method is still in use at some mills to partially refine low-grade bullion, but does not seem to be employed regularly in refineries. If the oxides of iron, &c., are not slagged off, the dross collects much gold which is difficult to separate by heat alone. If large quantities of base metals are present, the slag soon covers the surface of the metal, and prevents the access of the air. It would be possible to run off the slag and keep the surface of the metal clean as in cupellation, but it seems better to pass the air through the molten metal, as in the process next described.
In certain cases, the same object may be attained by granulating the bullion, roasting the granulations spread out on trays at a red heat, with frequent stirring, and melting the product with borax and sand. After two or three repetitions of such treatment, the bullion may be refined sufficiently to be sold.
Gold Bessemerisation
Bessemerisation is a new method, and consists in passing a stream of air or oxygen through molten bullion in clay pots by means of clay pipes similar to those used in Miller’s chlorine process. The base metals are oxidised successively in the order zinc, iron, antimony, arsenic, lead, bismuth, nickel, tellurium, copper. The oxidation of these metals, however, proceeds to some extent simultaneously, some copper being oxidised before the last traces of zinc are eliminated from the bullion. The oxides rise to the surface of the metal, and are slagged off with a mixture of borax and sand. Four parts of sand and three parts of borax are enough for slagging off about six parts by weight of base metals. Lead requires less than one- third its weight of sand, zinc and copper an equal weight of sand, and iron needs 1½ times its weight of sand. The borax may be in great part replaced by about half its weight of sand, but in that case the amount of iron in the slag should be kept greater than the amount of zinc. Tin oxide may be slagged off by pearl ashes. The zinc comes off first as a sheet of flame. Then sparks, due to the formation of magnetic oxide of iron, are seen above the charge. Afterwards the action proceeds more quietly. The end of the operation is difficult to determine, except by measuring the air passed through or by dipping out part of the metal, casting it, and bending the ingot. If it is tough, only gold, silver, and copper remain unoxidised. A little silver oxidises simultaneously with copper, and if all the copper is removed into the slag, from 10 to 50 per cent, of its weight of silver is also oxidised, and passes into the slag, from which it is recovered by fusion with carbon and iron. If the operation is stopped as soon as the metal is tough, the losses of silver in the slag are small. The losses of gold in the slag are insignificant, and bullion 990 fine in silver and gold may be obtained from metal only 500 fine in an hour or two. The slag prevents loss by projection or volatilisation, and the cost is trifling. This method is used at the Mint to toughen brittle standard gold.
Oxidation by Nitre
This ancient Gold Refining method is one in that the nitre must be “ projected ” upon the gold just before it melts, as it has little effect on molten gold. The method is still in wide use. The bullion is melted in clay crucibles, and a little nitre (potassium nitrate) or sodium nitrate is thrown on to the surface of the metal. Violent bubbling at once ensues, as heat converts nitrates into nitrites with evolution of oxygen. The nitre is pressed down with a stirrer, and the nitrites and undecomposed nitrates oxidise some of the base metals. The nitrates and oxides corrode the pots, and it is better to have a ring of bone ash next the pot, and to throw the nitre into the “eye” of metal in the centre. The oxides are absorbed by the bone ash, which protects the crucible from attack. After a minute or two, the action of the nitre moderates, and it is then, together with the bone ash, skimmed off with a ladle, and the operation repeated as often as necessary. If the metal is allowed to become pasty, so that it can be mixed with the nitre, the action is much more rapid and effective. If too much nitre is added at one time, the charge boils over, and part is lost.
Iron and zinc can be removed in this way, but the oxidation of lead is more tedious, and bismuth, tellurium, and copper are very troublesome. The losses by spirting are heavy, and large quantities of both silver and gold are entangled in the dross and skimmed off. In the treatment of cyanide precipitates the amount of these initial losses has been stated by Alfred James to be 10 per cent., and by J. S. MacArthur to be as high as 25 per cent. There is little to recommend the method, which seems to remain in use from force of habit. Potassium permanganate has also been used as a substitute for nitre.
Oxidation by Metallic Oxides
Black oxide of copper, CuO, was formerly used in certain cases. It was mentioned by Ercker. The oxide is stirred in with the molten metal, and the whole then allowed to remain in the furnace for about half an hour, with occasional stirring. All base metals are oxidised, the cupric oxide being reduced first to cuprous oxide, and then to metallic copper. The cuprous oxide is dissolved in the metal, and so carries oxygen to all parts of the molten mass. The process is efficacious, but the gold is, of course, contaminated with the reduced copper.
The use of manganese dioxide in the Transvaal in refining gold-zinc-slimes from the cyanide process was described by Johnson and Caldecott in 1902. As a cheaper alternative, the author proposed ferric oxide, but this has apparently not been tried. In these cases, the oxides are reduced to lower oxides, but not to metals, so that the bullion is not contaminated by the products.
Chlorination
Sal-ammoniac, NH4Cl, is sometimes used to remove lead from gold bullion. When much lead is present, alternate additions of nitre and sal-ammoniac have been recommended. It is probable that the sal-ammoniac acts by decomposing basic compounds of lead which resist the action of nitre. Cupric chloride acts like gaseous chlorine, chloridising base metals and being reduced to cuprous chloride which is volatilised. The fineness of bullion is slightly raised by this agent, but it is not suitable for ordinary refining, although it may be used for toughening brittle standard gold or high-grade bullion containing traces of impurities. Gaseous chlorine is better. Its use is described under the heading of Miller’s process of Parting, p. 407. In 1870, 40,000 ozs. of brittle standard gold were toughened at the Mint by the use of chlorine gas. The charges were about 1,100 ozs. each, and the time of passage of the chlorine varied from five to seven minutes. The amount of impurities removed was from 0.1 to 0.3 per 1,000.
An old method of removing traces of impurities from brittle gold was to make repeated small additions of powdered corrosive sublimate (mercuric chloride). After each addition the door of the furnace must be at once closed, as dense poisonous fumes arise and must not be breathed by the workers. Volatile chlorides of zinc, copper, antimony, bismuth, &c., are formed and pass off, carrying with them some gold, of which there is an appreciable loss. A little corrosive sublimate sprinkled on the surface of molten gold will completely toughen every part of it without being mixed with it by stirring, even although the crucible contains several hundred ounces of the metal.
In 1866, 8,800 ozs. of brittle standard gold were toughened in this way at the Mint. It was divided into 14 charges of about 650 ozs. each, and from 12 to 16 ozs. of corrosive sublimate were added to each pot, the total amount used being 182.3 ozs. troy, or about 2 per cent. The tough bars produced weighed 8,762 ozs., the average fineness being 918.9, or 2.3 per 1,000 higher than the brittle bars. The actual loss of gold was 6.657 ozs., or 0.756 per 1,000.
Gold Refining by Sulphurisation
Sulphurisation is said to be practised in the United States Mints; the following is a brief account: It is effected in plumbago crucibles, and has for its main object the elimination from retorted metal of iron, when, as sometimes happens, it is present in large quantities. The metal is kept just above its melting point, the temperature being as low as possible in order to avoid unnecessary waste of sulphur by volatilisation. Sulphur is sprinkled round the edges of the molten mass, and stirred in with a graphite stirrer. If sulphur is added near the centre, particles of gold are lost by projection. Sulphide of iron is formed with great energy, and sulphide of silver also, but the latter is not produced rapidly until nearly all the iron has been already converted into sulphide. The gold is unaffected by the sulphur and subsides to the bottom. It is not usually cast by pouring, but allowed to solidify in the pot, a better separation between the gold and the matte being thus effected. The pot is turned out as soon as solidification has taken place, and the matte is broken off by a hammer, the gold being remelted and cast into a bar. The small quantity of gold taken up by the matte is separated by melting with metallic iron.
When retorted metal is infusible from the presence of large quantities of iron free from carbon, it may be refined by melting with galena, according to H. L. Sulman with pyrites, according to T. C. Cloud ; or with sulphur.
How to Use Iron and Carbon to Refine Gold
Iron is used to remove arsenic, antimony, sulphur. The pot is stirred briskly with an iron rod for a few minutes. Antimonides, arsenides, or sulphides are formed and separate from the metal. Carbon is used to assist iron to melt and to remove oxygen from bullion.
At the Mint it is found worth while to recover the gold from the iron tools used in stirring, dipping. For this purpose they are melted down in a graphite crucible with a little charcoal to make grey-iron, and kept at a white heat for some time, after which the charge is allowed to cool slowly. Under this treatment the gold and silver separate out (an alloy containing three or four parts of silver to one part of gold being better for the purpose than pure gold), and are found at the bottom of the crucible sharply marked off from the surface of the iron, which is now quite freed from the precious metals.
The melting under charcoal is sometimes necessary to render silver bars fit for coinage when they have been treated by nitre. When silver has been raised to a high degree of fineness, it is affected by a peculiar bubbling due to the evolution of oxygen previously absorbed from the nitre. In this case it is necessary to stir continuously with a graphite rod, keeping the surface covered with charcoal powder, until the bubbling ceases. If the metal, while still effervescing, is poured into a mould, it sprouts at the surface, and a shower of extremely minute particles are projected, often to some distance from the mould, requiring to be swept up; if the crucible is covered by a lid, very heavy effervescence ensues when the lid is lifted. In this case the silver ingots formed are not marketable, being brittle, of low density, and covered by heavy efflorescences.