Table of Contents
The following gold leaching test procedure has been found satisfactory for carrying out cyanide leaching tests by agitation. Winchester bottles of about 2.5- to 4-liter capacity are used. It is convenient to number the bottles and determine their tares, etching the figures on the bottle by means of hydrofluoric acid and then marking over the etching with a china or glass marking pencil. When a wet pulp such as the tailing from amalgamation or concentration tests is the material to be tested by cyaniding, the weight of the pulp is obtained by weighing bottle and contents and subtracting the tare. When the test is finished, the solids are dried and weighed, and the amount of solution used may be determined. Otherwise a weighed amount of ore is put into the bottle, and then a weighed amount of lime of known available lime content is added. Next is added a measured amount of a cyanide solution of known strength. The bottle with the pulp is then agitated.
The agitating device (Fig. 4) consists essentially of a series of horizontal, rubber-covered, wooden rollers about 4½ in. in diameter and 30 in. in length, and mounted on bearings with their axes parallel. They are spaced at about 7-in. centers and rotate at 50 to 75 r.p.m. When a bottle containing pulp is placed between two adjacent rollers, it is caused to revolve and the pulp is agitated. It is convenient to have a board set over the ends of the rolls and at a right angle to their axes with a notch midway between the centers of the rolls to engage the neck of the Winchester bottle.
The notches are at such a height that the bottles are tilted, allowing them to be filled with more pulp than if they rested on their sides and with no danger of the pulp’s splashing out.
Agitated Gold Leaching Tests
Enough ore for the maximum probable number of tests is prepared by dry grinding so that it all passes a certain size, say 100 mesh. Samples for assay and screen analysis are taken.
We shall assume that it is desired to determine the extraction obtained by treating a certain ore with a solution containing 0.05 per cent sodium cyanide, NaCN, and 0.025 per cent CaO (protective alkalinity), which means that the solution is to contain 1.0 lb. NaCN (free cyanide) and 0.5 lb. CaO (protective alkalinity) per ton of solution. The pulp is to be agitated at a dilution of 2.5 parts of solution to 1 part of ore for 24 hr.

FIG. 4. A device used in the testing laboratory of the Dorr Company for making bottle cyanide agitation tests. As many as 20 bottles can be rotated at one time.
All crude ores will be found to vary in their lime-consuming power, and the extent of this factor should be determined prior to treatment. Provision must be made for the addition of enough lime not only to satisfy the lime-consuming power of the ore but also to produce the proper hydrogen- ion concentration during treatment. In determining the lime-consuming power of raw ores, the following procedure has been recommended by LaMotte, Kenny, and Reed in their book pH and Its Practical Application.
Determine Lime Comsumption of an Ore
Fifty-gram samples of the dry, ground ore are placed in 8-oz. bottles with 200 cc distilled water. To the first sample bottle no lime is added, to the second the equivalent of 0.5 lb. calcium oxide per ton solution is added, and to the third, fourth, fifth, and other bottles 1.0, 1.5, 2.0, etc., lb. calcium oxide per ton solution is added. The bottles are stoppered and agitated for 1 hr., after which the pulp is filtered and the pH value of the filtrate determined. An analysis of the filtrate is then made to determine the amount of calcium oxide remaining in solution. When this value is known, the amount of lime consumed by the ore can be calculated. This procedure permits excellent control of the regulator, not only giving greatest economy but at the same time enhancing the efficacy of the other reagents used.
A 400-gram sample of this ore is weighed out and put into a bottle. (Four hundred grams is a convenient amount, although any amount from 100 grams to 10 kilograms may be taken, bottles of suitable size being used.)
Add lime to gold leaching test
It will be assumed that preliminary tests or other information indicate that the ore will require about 2.0 lb. CaO (100 per cent basis) per ton for neutralization during the first hour of agitation. This means that 400 grams will require 0.4 gram CaO. In addition to the lime for neutralization, enough lime will be needed to bring the solution to 0.025 per cent CaO. At a dilution of 2.5 to 1 there will be 1000 cc (or ml) of solution which, at 0.025 per cent CaO, will require 0.25 gram. The total lime to be added then is 0.40 + 0.25, or 0.65 gram. The hydrated lime has been tested and found to contain 69.0 per cent available CaO; therefore, we shall add 0.65/0.69 = 0.94 gram of hydrated lime.
Water is added next, and the amount is determined as follows: 400 grams of ore requires 1000 cc of solution for a dilution of 2.5 to 1; 1000 cc of solution at 0.05 per cent NaCN contains 0.5 gram NaCN, equivalent to 50 cc of the stock 1.0 per cent NaCN solution. Therefore, add 950 cc water.
The pulp is shaken in the bottle for a moment, and then 50 cc of the 1.0 per cent cyanide solution is added.
Sometimes it is desirable to check the dilution, and this may be done readily by weighing bottle and contents if the tare of the bottle is known.
The bottle is now placed on the rollers and agitated, the time being noted. At the end of a period varying from ½ to 2 hr., depending upon the rapidity with which the cyanide or the lime is consumed, the solution is titrated for free cyanide and protective alkalinity.
This is done by removing the bottle from the rollers and allowing the pulp to settle until some of the supernatant solution can be drawn off in a pipette. If the solution is clear enough, a 10- or 25-cc sample may be cautiously drawn off in a pipette and titrated direct. If the solution is turbid, about 50 or 60 cc may be drawn off, using a 100-cc pipette. The solution is filtered through a dry filter into a clean, dry beaker, and a sample is taken for titration.
How to Determine the Cyanide and Lime Consumption
The following notes of a typical test will illustrate the method of calculating the consumption of cyanide and lime:
- Ore—400 grams.
- Lime added—0.94 gram hydrated lime = 0.65 gram CaO.
- Solution—1000 cc (950 cc H2O, 50 cc 1.0 per cent NaCN solution).
- Solution strength to be maintained at about 0.05 per cent NaCN and 0.025 per cent CaO.
9:00 A.M.—start
10:00 A.M.—25 cc taken out for titration, giving 0.042 per cent NaCN, 0.022 per cent CaO.
The solution is now brought back to a volume of 1000 cc having a strength of 0.05 per cent NaCN and 0.025 per cent CaO.
975 cc at 0.042 per cent NaCN = 0.41 gram NaCN; 1000 cc of solution should contain 0.50 gram NaCN; therefore, add 0.09 gram NaCN or 9 cc 1 per cent NaCN solution and 16 cc H2O.
975 cc at 0.022 per cent CaO = 0.21 gram CaO; 1000 cc of solution should contain 0.25 gram; therefore, add 0.04 gram 100 per cent CaO or 0.04/0.69 = 0.058 gram hydrated lime.
1:00 P.M.—25 cc taken out for titration, giving 0.048 per cent NaCN and 0.02 per cent CaO.
The cyanide strength is close enough to that desired so that no correction is necessary. The protective alkalinity, however, is lower than desired and is raised as follows:
975 cc at 0.02 per cent CaO = 0.195 gram CaO. This amount subtracted from 0.250 gram (1000 cc at 0.025 per cent) = 0.055 gram; therefore, 0.055/0.69 or 0.08 gram hydrated lime is required. As the pulp may be expected to continue to consume lime for the next few hours, a slight excess over the theoretical amount may be added, say a total of 0.10 gram. Then, 25 cc of water is added to bring the volume of solution back to 1000 cc.
9:00 P.M.—25 cc taken out for titration, giving 0.045 per cent NaCN and 0.023 per cent CaO.
To bring the solution to the desired strength there are required 0.50 – 975 x 0.00045 = 0.06 gram NaCN and 0.25 — 975 X 0.00023 = 0.026 gram CaO. As the pulp is now to be left to agitate overnight without further attention, add slightly more than these amounts to take care of consumption, say 0.08 gram NaCN or 8 cc 1.0 per cent solution and
0. 05 gram hydrated lime. Also added 25 minus 8 or 17 cc water to bring the solution volume to 1000 cc.
9:00 A.M.—finish of 24-hr. agitation. The solution titrates 0.049 per cent NaCN and 0.023 per cent CaO.
Results may be calculated as follows:
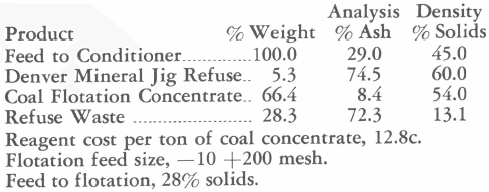
It may not be necessary or particularly informing to carry out tests to such a detailed degree as that shown. Each experimenter may determine for himself the extent to which it is desirable to correct solution strength and dilution during testing.
Rather than maintain the solution strength at approximately a predetermined figure, some experimenters prefer the more simple method of not making corrections of the solution during the period of agitation, starting with a solution high enough in cyanide and lime so that at the end of the period the solution is considered to be adequate. Such a procedure, however, is open to objection on the ground that the solution may be too strong at the beginning and too weak at the end, and these conditions may lead to erroneous deductions.
Series Gold Leach Testing
After a preliminary test has indicated the consumption of cyanide and lime, several series of tests may be outlined in which the effect of changing one variable is determined. These variables may be cyanide strength, protective alkalinity, fineness of grinding, time of agitation, dilution, addition agents (lead of mercury salts, bromocyanide. and others).
Each series should embrace 4 to 6 deg. of the variable or as many as may be believed to be desirable. Finally, several tests may be made using various combinations of the best conditions as determined by the series tests.
Addition of Gold Leaching Agents
With certain ores it may be found that the addition of a small amount of a lead or mercury compound is beneficial, either in purifying the solutions or in increasing the extraction or both. Lead in the form of litharge, as the nitrate or as the acetate, and mercury as the metal or oxide may be used. Mercury is now seldom used.
It is always worth while to make parallel tests with and without one of these agents. One-half to 1 gram of litharge or 4 or 5 grams of mercury added to 400 to 1000 grams of ore is ample. In practice, probably less than ¼ lb. of litharge per ton of ore is generally enough. As the effect of litharge may vary greatly with the amount used, it is of great importance to determine accurately the critical amount to use.
Sometimes it is desired to determine the effect of bromocyanide on certain gold ores, particularly those containing tellurides. As bromocyanide is an unstable compound, it must be freshly prepared before use, and as it and its vapors are extremely poisonous, due care should be used in its preparation and use. When bromocyanide is being used, the protective alkalinity should be kept at the lowest possible point. In this connection a parallel test of the same low alkalinity but without the bromocyanide should be made to make sure whether possible improved results should be ascribed to bromocyanide or to low alkalinity. The reason for maintaining a low alkalinity when bromocyanide is used is because BrCN is rapidly decomposed by alkali. The reaction is usually illustrated thus:
BrCN + 2KOH = KBr + KCNO + H20
In his Manual of Cyanidation, E. M. Hamilton says this:
The usual method of making the reagent for laboratory use is to add a strong solution of cyanide to bromine (and not conversely) until the brown color is just discharged:
KCN + Br2 = KBr + BrCN
The quantity of BrCN may be determined in a working cyanide solution by acidifying with hydrochloric acid, adding an excess of potassium iodide and titrating the liberated iodine with decinormal sodium thiosulphate. J. E. Clennell in his Chemistry of Cyanide Solutions, gives the following reactions:
BrCN + HCl = HCN + ClBr
ClBr + 2KI = KCl + KBr + I2
1 cc 0.1N thiosulphate = 0.00529 gram BrCN
The BrCN is added to the cyanide solution to be used for extraction purposes in the proportion of about 1 BrCN by weight to 4 of KCN.
Change of Gold Solution
It is often of interest to determine the effect of a change of solution upon the extraction (rate and amount) and the chemical consumption. The procedure of effecting such a change will depend primarily upon the dilution of pulp during agitation and the dilution to which the pulp will settle after standing a short time. It is preferable to remove the solution by decantation rather than by filtration.
Precise manipulation is necessary in effecting a change of solution. Deductions as to the effect of this procedure should be made with caution, the practicability of reproducing the effect in plant operation being kept in mind.
Grinding in Solution
The small samples available for laboratory experiments cannot be expected to reproduce exactly conditions that will exist in large-scale milling practice. Actual size reduction of the ore can be duplicated, of course, but closed-circuit grinding with a high circulating load would be very difficult to reproduce on a laboratory scale. Particularly is this true when grinding in cyanide solutions is to be practiced.
This is not to say that grinding in cyanide solutions should not be performed in the laboratory. On the contrary, such grinding is likely to add considerably to the knowledge accumulated in testing, and it should be done. But the data thus gathered should be weighed with mental reservations and regarded as subject to some variation. As a matter of fact, laboratory grinding in solution is standard practice at some testing plants, and the information gathered is regarded as valuable.
Perhaps the nearest approach to solution grinding in the laboratory is to grind in water, classify to desired sizes, and settle. Then cyanide solution and lime are added to make up a solution of the desired strength. This pulp may be then agitated for a time, ½ to 1 hr., to check the approximate dissolution in the mill-classification circuit. After this the pulp may be thickened to the dilution at which the agitation and aeration are to take place.
Cyclic Use of Solution. After preliminary batch tests have indicated the optimum conditions such as fineness of grind, dilution, strength of solution, and time of agitation, tests should be made in which cyclic operations of agitation, thickening, decantation, washing, and precipitation are carried out. The important point here is the reuse of the solution. It may be found that fouling of the solution takes place and its efficacy diminishes to a greater or lesser degree. If such be the case, it is essential that some method of reactivating or purifying the solution be determined to avoid the necessity of discarding too large an amount.
Testing for Gold Reprecipitation
Reprecipitation of the dissolved gold may under certain circumstances take place in the ore pulp before the solution is separated from the solids. The most frequent cause is the presence of carbonaceous material in the ore, but it is not generally realized that pyrite and pyrrhotite in the presence of low free cyanide concentration can also act as effective precipitants for gold.
A suitable test is to agitate 500 grams of the ore with a pregnant solution containing a low cyanide concentration and the same amount of gold as contained in 500 grams of the ore. It was found, for instance, in the case of one ore tested by this method that 85 per cent of the gold was precipitated from the pregnant solution by the ore.
