When precipitation is complete the precipitate must be separated from the solution. The means of bringing about this separation will be described under this head. Under the next head (Washing) the completeness of this separation will be considered.
Filtering Medium
In quantitative gravimetric analysis the two materials generally used are paper and asbestos, and the student must be familiar with the use of both of these. He has already used paper in his qualitative work, but in quantitative analysis paper of a special quality must be used. This paper in various qualities may be obtained from the dealers. It is usually put up in packets of 100 pieces of circular shape. The most convenient sizes are 9 cm. and 11 cm. in diameter. As these papers are frequently incinerated along with precipitates, it is obvious that any ash resulting from the incineration of the paper must be allowed for. The amount of ash in each paper is generally stated on the cover of the packet, but this should be confirmed by the method given under “ Ignition.” For the present it will be assumed that the student has procured two packets of papers of the quality and size mentioned.
Papers of the sizes mentioned should not yield more than one milligram of ash. Papers specially treated with hydrochloric and hydrofluoric acids may be obtained. In these the ash which is less than 0.0003 gm. may be disregarded. If time permits, the student may prepare these papers. Papers of 7 cm. diameter will be found convenient if very small precipitates have to be filtered, or if the bulk of the filtrate has to be kept as small as possible. A small paper can be washed with less solution than a large one. Besides paper, the chief filtering medium in extensive use is asbestos. This in conjunction with the Gooch crucible gives the most convenient, accurate, and rapid method known of filtering many precipitates. Paper consists largely of carbon, which on incineration passes away as CO2, leaving a small quantity of ash behind. Asbestos, on the other hand, when properly prepared and incinerated, suffers no loss. When, as in the Gooch method (see Methods of Filtration), a precipitate is separated on a thin layer of asbestos, it may then, without additional handling, be dried and incinerated or it may be dissolved even by strong acids. In his future work the student will see that in many cases, both as regards accuracy and convenience, asbestos is preferable to paper.
In order that asbestos may give accurate results, it must be unacted on by the acid or other substances present in the solution and subsequent washings, and must suffer no loss on ignition. The asbestos chosen should be of a soft silky fibre. A little is taken in the fingers and carefully scraped down with a knife into very fine soft down. Continue the process till sufficient is obtained for a number of filtrations. Transfer the fine asbestos to a glass beaker, add strong HCl and boil for half an hour or longer. Decant the greater portion of the acid, add distilled water, and transfer the contents of the beaker to a large filter (either of paper or the platinum cone). The asbestos is then thoroughly washed, then dried in the air oven and transferred to a bottle for safe keeping. The preparation of the filtering surface will be described under the Gooch method of filtration.
Methods of Gravimetric Precipitate Filtration
In analytical work the separation of the precipitate from the solution is effected by passing the solution through some medium the pores of which are sufficiently fine to obstruct the passage of particles of the precipitate. Generally the solution passes through from above, and the weight of solution above the filter causes the solution to run through. The rate of filtration may be much increased by the use of a vacuum underneath the filter, which may be either paper or asbestos. The student must be proficient in both methods.
Filtration unaided by a vacuum
This method has frequently been used by the student, and the necessary apparatus is by this time quite familiar to him. It may happen that in his qualitative work the student has acquired careless habits. If, on reading the following, he finds that his practice does not coincide with the details laid down, he must reform his ways. It is only by continuous attention to details that the skill in manipulation which marks a good chemist can be secured.
The necessary apparatus is shown in fig. 16, and consists of beakers, funnels and stand, glass rod and filter papers.
Carefully fold one of the papers twice at right angles, as shown in fig. 15, p. 5. Place it in a funnel (sides sloping at 60°), carefully opening out and pressing the paper to the glass. Place the funnel and paper on the stand. Place a beaker under the funnel and adjust its height so that the tip of the stem touches the edge of the beaker near the top, so that the filtrate may run quietly through and not splash. Now carefully spray the paper with hot distilled water from the wash bottle. Take the beaker with the precipitate to be filtered in the right hand and the glass rod in the left, and held at its upper end in the tips of the fingers and thumb. Bring the lower end within half an inch of the filter paper near the point, and carefully pour the liquid down the glass rod by bringing the lip of the beaker in contact with the rod. The solution is run in till 1 or 2 cm. from the top. Remove the beaker, letting any drop on the lip of the beaker run down the rod (not the outside of the beaker) into the filter. When the bulk of the liquid has filtered through, the funnel is replenished and the process repeated till the contents of the beaker have been transferred to the filter. The final stage of this transference is effected by the wash bottle, the beaker being nearly inverted, with its lip over the filter, and the jet of the wash bottle at the same time directed with the finger upwards into the inverted beaker, and any adhering precipitate is washed down on to the filter. If the precipitate is to be washed by decantation (see Washing), it is not at this stage transferred to the filter paper. The method described is that generally adopted where paper is used as the filtering medium. It answers well with substances that filter easily and do not choke the pores of the paper, but with many substances it leaves much to be desired as regards time.
Filtration accelerated by a vacuum
As has just been mentioned, filtration becomes prolonged and tedious in certain cases, and any moans of cutting down the time is of value both to the student and the practising chemist. The usual method of accelerating filtration is by the application of an exhaust, that is, by the creation of a vacuum on the under side of the paper or other medium. The means of creating this vacuum and connecting it to the filter will first be considered, then the methods of accelerated filtration through paper and asbestos. Where a water supply at a fair pressure is available, the most convenient method of obtaining an exhaust is by what is termed a filter pump. This is simply a small injector, working on the same principle as the boiler injector (steam). The water, under pressure from the tap, forcing its way through the conical jet opening, forms a vacuum, and the air is sucked in at the side. If rubber connections are used, they must be firmly bound and wound with copper wire. Metal connections are preferable, though rubber will do. A rubber tube is connected to the side tube, and at the other end to a flask or Woullfs bottle, as shown in fig. 52. This prevents any water, running back from the pump (on turning off the suction), from running into the filtrate.
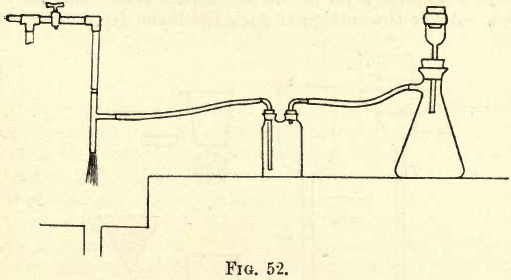
Whenever the aid of the vacuum is required, a connection is made between the Woulff’s bottle and the filtering apparatus, which is shown on the right hand in the sketch.
Accelerated Filtration through Paper
Unless strengthened, the usual filter paper is rather too weak to stand the extra pressure caused by the suction, therefore a small platinum cone, thickly pierced with small holes, bored from the inside outwards, is used in the apex of the funnel. This cone may be made from platinum foil. The cone is dropped into the point of the funnel; then the paper is fitted in; the funnel stem is passed through a cork which fits the mouth of the filter flask. The cork and funnel are firmly fixed in the filter flask, and its side branch is connected to the Woulffs bottle. Fill the funnel with water, and start the pump to see that everything is in working order. If so, empty the water out of the flask and then commence filtration, always keeping the funnel well filled with the liquid. The exhaust should always be turned on gradually, and worked up to its full capacity only when necessary. The filter flask may be conveniently replaced by a separating funnel if it is desired to draw off the filtrate during the process.
Accelerated Filtration through Asbestos—Gooch Crucible & Platinum Cone
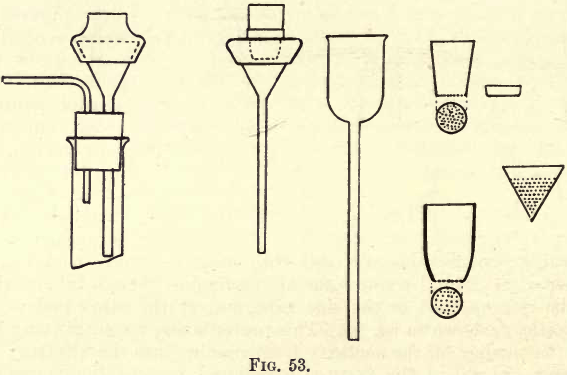
The preparation of the asbestos fibre has been described. The necessary apparatus and method of filtration will now be taken. The crucibles used are shown in fig. 53.
They may be made either of porcelain or platinum. Platinum is more satisfactory, but more expensive. The bottom of the crucible is pierced by a large number of fine holes. The crucible is fitted into the glass funnel (see fig. 53) by a rubber joint consisting of a 5 cm. length of wide bore, thin walled, rubber tubing. An air-tight joint is thus secured. The funnel stem, passes into the filter flask as usual, and the usual arrangements connect the filter to the exhaust. The platinum cone shown may be used in a funnel, the subsequent operations being the same as those with the crucible, except that the cone cannot be used for ignitions. Having now connected up the apparatus, the asbestos filter must be prepared. Take from the bottle a little of the asbestos, place it in a beaker, and stir it up with 50 to 100 c.cs. distilled water; pour slowly into the crucible and turn on the exhaust. Continue pouring in the solution down a glass rod, the tip of which is kept near the bottom of the crucible. A fine coat of asbestos gradually forms. A little more asbestos is added in the same manner till a uniform layer about the thickness of moderately stout filter paper is obtained. Allow the suction to suck the layer dry.
The crucible is now ready for filtration, but as it will also be used for ignition and weighing of the precipitate, it is now removed. Any fibres adhering to the bottom are removed and the small cap is slipped on. (These caps may be procured in platinum for porcelain crucibles, and are an advantage in incineration.) The crucible is now dried in the water oven, then incinerated and weighed (see following headings). The cap is now removed and the crucible replaced in the funnel, and filtration commenced by gently pouring the solution down a glass rod, the tip of which is brought close to the asbestos. The vacuum is then applied as usual.
This method of filtration is specially applicable when the precipitate is to be ignited. It is also of much value when a precipitate is to be dried and weighed without ignition. The method of weighing such a precipitate on counterpoised or weighed filter papers is inaccurate, and should always be replaced by the Gooch method.
The necessary apparatus is cheap if a porcelain crucible be used. A small thin platinum cap is cheap, and may be slipped on the porcelain during ignition, though the ignition may be performed without this device.
