This Gutzkow Process of parting by sulphuric acid was invented and patented by Mr. F. Gutzkow. It has been extensively worked in Germany and in San Francisco, and up to the year 1891 had been instrumental, on the authority of Mr. Gutzkow, in refining one hundred million dollars’ worth of silver. It is fully described in Percy’s Metallurgy of Silver and Gold, and only a brief account will be given here. When the patent had expired, Mr. Gutzkow introduced and patented several improvements on it, which were used for some time at the Consolidated Kansas City Smelting and Refining Company’s Works at Argentine, Kansas, in 1892, but subsequently abandoned, at least in part.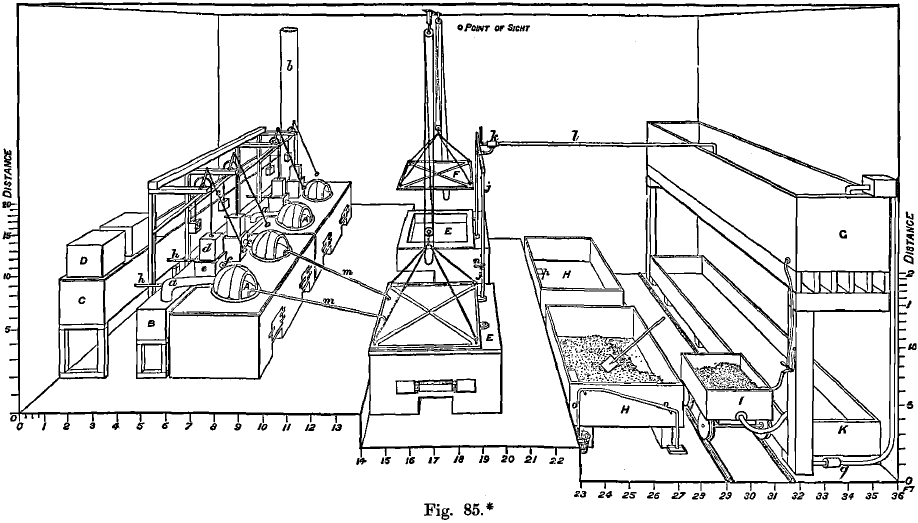
The original Gutzkow process, as employed at the San Francisco Assaying and Refining Works for many years, may be summarised as follows:—The bullion treated is of three kinds:
- Gold bars from retorted metal, containing about 900 parts of gold, 10 to 20 of base metals, and the remainder silver;
- Comstock silver bars or dore bars, usually containing 20 to 100 parts of gold per 1,000;
- base bars from the Reese River district and from pan-amalgamation of tailings, containing from 100 to 800 parts of silver, and the remainder chiefly copper, with sometimes a little gold.
The gold bars (1) are alloyed with silver and granulated, but the others are cast into bars, and parted in that form. The dore bars, when prepared for solution in the acid, weigh about 100 lbs. each, and are 12 inches long, 6 inches broad, and 5 inches thick. The base ingots are melted with fine bars to reduce the average copper contents to 12 per cent., and are cast into bars 1 inch thick, the gold from which is only about 992 fine.
The boiling is done in flat-bottomed thin cast-iron kettles (A, Fig. 85), of which the bottom is only ¾ inch thick when new, and ¼ inch when worn out. The solution can be rapidly heated, owing to the thinness of the iron kettles, and 200 lbs. of alloy are dissolved in four hours by means of 300 lbs. of sulphuric acid, which comes from the tank, C, and is forced into the kettle through the pipe, f by the plunger, d. The solution is then siphoned off through the pipe, m, into the tank, E, and diluted with a large quantity of hot mother liquor from a previous crystallisation, which is mainly sulphuric acid of about 58° B.; some water is also added, and the solution partially cooled, so that some crystals of silver sulphate are enabled to separate out and carry down with them the milky precipitate of lead sulphate and any suspended particles of gold; green basic sulphate of iron also settles firmly. The clear solution is then siphoned off into H and cooled to 80° F., and almost all the silver sulphate thus crystallised out. If the acid is concentrated, white soft crystals of bisulphate are formed, which is not desired ; if, however, the acid is only at about 58° B., large hard yellow crystals of monosulphate, free from acid but contaminated with copper, are deposited. The mother liquor is pumped back into the tank, E, or to the original acid tank, the device employed for this purpose being to exhaust them of air, so that the acid is sucked up without passing through any valves, which would soon wear out. The crystals of sulphate of silver are transferred to the filtering box, I, by iron shovels, and a hot solution of green vitriol of 25° B. run on to them from G. This is at first mainly occupied in dissolving the sulphate of copper, and the first portion of the solution, after passing through the filtering box, is run into a storing vat, where the silver, incidentally dissolved, is precipitated by copper, and the latter subsequently recovered by means of iron. After a time, the copper being dissolved, the silver begins to be reduced, the green solution of iron turning coffee- brown ; the reaction is as follows :
2FeSO4 + Ag2SO4 = Fe2O3. 3SO3 + 2Ag
The reduction may also be affected by sheets of metallic iron, which is first converted into ferrous sulphate and then into ferric sulphate, the silver being simultaneously reduced to the metallic state. The brown solution of ferric sulphate is boiled with metallic iron in K, in order to regenerate the ferrous salt. The silver is washed, pressed, dried, and melted. The gold from the original dissolving kettle is also washed in a filter, pressed, dried, and melted.
Such was the original Gutzkow process as employed in treating dore bars. Its chief advantage over the ordinary sulphuric acid process was the saving of acid. In the ordinary process, none of the acid used is saved, so that it is reduced in amount as much as possible, but does not fall below twice the weight of the silver dissolved. This reduction in the amount of acid used makes the finishing of the dissolving a difficult and delicate operation. In the Gutzkow process, however, only the acid decomposed by the silver is lost; the weight of this is about equal to that of the metal, the rest of the acid being all recovered and used over again in the boiling. Moreover, the long and tedious crystallisation of copper sulphate is avoided, and the space required for the crystallising vats saved. However, several large lead-lined vessels are required for the storage of the various solutions, and the expense of these, as well as the space required, is greatly reduced by the recent improvement described below.
The New Gutzkow Process
Mr. Gutzkow has pointed out that if a large amount of acid is used for the boiling, not only is the silver more completely dissolved and the operation greatly expedited, but the presence of a high percentage of copper does not hinder the parting, as it is kept in solution by the excess of free acid. Thus, for ordinary dore silver, he proposed to use four parts of acid to one of bullion; for bars containing 20 per cent, of copper he uses six parts of acid ; for still baser bullion, more acid, and so on, never losing more than one part of acid for one of bullion, and recovering the remainder.
The charge for a pot 4 feet in diameter and 3 feet in depth is 400 lbs. of dore silver: the pot is flat-bottomed, with a basin-shaped pocket or well in the centre which is useful for the collection of the gold. The bullion is first attacked by fresh acid of 66° B., run in by gravity from a large tank, and, when most of the silver has been dissolved, mother liquor from a former operation is added, a pitcher-full at a time, until the charge is completely dissolved, which takes from four to six hours. The fire is then moderated and the pot filled with mother liquor to within 1 or 2 inches of the top, when the temperature of the acid will have been so far reduced that only faint fumes are discernible. If no fumes are visible the acid is too cold and some silver sulphate will be precipitated, but otherwise the large excess of acid will keep it in solution. The well-stirred charge is now allowed to settle, which is perfectly accomplished in ten minutes, as the yellowish slowly-subsiding persulphate of iron is transformed to a greenish flocculent compound by the water in the mother liquor, and this settles quickly and carries all suspended matter to the bottom. More iron is dissolved from the kettle than in the ordinary process, owing to the greater dilution of the acid used in boiling.
The solution is now siphoned from the kettle by means of a ¾-inch gas pipe into a large cast-iron vessel, only about 1 foot deep, standing in a larger vessel which can be filled with water for cooling the charge. Steam is blown into the still hot acid solution through a lead nozzle, 1/8 inch in diameter, pointing vertically downwards. This both dilutes and warms the solution, the heating being necessary in order to prevent crystallisation of the silver consequent on the dilution. As soon as the dilution has proceeded sufficiently far to ensure the crystallisation of the hard yellow monosulphate instead of the soft white bisulphate of silver, a point which is found by dipping out small quantities at intervals, and observing their behaviour on cooling, the steam is shut off and the vat cooled with water and left all night. The silver crystals form a coating of about 1 inch thick, which is contaminated with copper sulphate if the mother liquor, by repeated use, has become saturated with it. The mother liquor is now pumped back into the acid storage tank by the creation of a vacuum, and the crystals of sulphate of silver are detached with an iron shovel and thrown into a filtering-box provided with a false bottom. Cold distilled water is sprinkled on the charge, and is allowed to filter through it and flow back into the crystallising vat, until the greater part of the free acid has been removed. The stream is then deflected into a “ silver filter ” where any silver is precipitated that may have been dissolved at the same time as the sulphates of iron and copper. The silver filter is a lead-lined box, partly filled with precipitated copper and provided with a false bottom. The silver separates on the top of the copper as a spongy sheet, a corresponding amount of copper being dissolved. When the crystals of silver sulphate in the first-named filtering box have been completely freed from acid, and from copper and iron sulphates, the stream of water is discontinued. The spongy sheet of silver is then removed from the “ silver filter ” box and treated with hot water and a few crystals of silver sulphate to dissolve the copper still retained by the sheet. During this whole operation of sweetening the crystals of silver sulphate, only about 3 per cent, of it is dissolved, as it is little soluble in cold water. The copper solution, after passing through the “ silver filter ” is either run to waste or precipitated by scrap iron in wooden tanks at a nearly boiling temperature.
The crystals are now dried in an iron pan which is placed above a furnace, and, after being mixed with about 5 per cent, of charcoal, they are at once charged into a hot crucible in a melting furnace. The silver sulphate is reduced at a low red heat to metallic silver, carbonic and sulphurous acid gases being evolved. By the time the temperature of melting silver is reached, these gases will have all passed away. The silver is toughened by adding nitre and borax until the so-called “ boiling ” indicates that the sulphur has all been eliminated, and the metal is then cast into bars.
The gold residue in the dissolving kettle contains insoluble sulphates of lead, iron, antimony, mercury, and often some copper and silver. It is ladled out and boiled with water to dissolve out the sulphates of silver, copper, iron, &c., and, after thorough filtering, it is stirred in a dish with hot water, and decanted on to a filter-cloth until the insoluble sulphates of lead, &c., have all been washed off, and the gold is left bright and clean. The gold is stored until enough is collected to make a 200-oz. bar, which is usually brittle. The material collected on the filter-cloth is re-washed once or twice to recover the particles of gold from it, and can then be reduced with charcoal and cupelled. If lead is present in the original alloy, part remains with the gold, and is dealt with in the manner which has been already described, but the greater part is carried off with the silver solution, and is deposited both while the steam is being passed in, and also subsequently during crystallisation of the sulphate of silver, which is coated with it. The sulphate of lead is removed from the crystals by stirring them well in a stream of cold water, by which the light insoluble particles of lead and antimony sulphate are carried away; it can then be collected, reduced and cupelled. Any silver that may be dissolved in the course of this washing is precipitated by copper as before.
The process is seen to differ from the original one in three essential particulars:—1. The solution is diluted with steam instead of with mother liquor, the amount of liquid in use, and consequently the number of lead-lined vats required being thus reduced. This is an important item, especially in the United States where lead-burning is expensive, owing to the existence of a powerful union. 2. The weak silver solution is precipitated at once, instead of being stored in tanks to be used again or to be precipitated at leisure. 3. The silver sulphate is reduced directly with charcoal in a crucible in the furnace. This saves the pressing of the silver and, what is of greater importance, avoids the use of the solution of sulphate of iron, which needs to be stored. The reduction in the crucible and subsequent melting requires scarcely more fuel than would be used to melt the pressed silver. One of the minor advantages of the process is said to be that no stirring is required during the boiling, owing to the large amount of acid used. This saves labour and enables the acid fumes to be more easily condensed, as they are not mixed with air, which in the ordinary way would enter through the aperture left for stirring. The exclusion of air also helps to prolong the life of the iron kettles by checking the attack on them by sulphuric acid.
Titus Ulke, however, announced that the method of reducing sulphate of silver by heating it with charcoal, was tried by the Consolidated Kansas City Smelting and Refining Company, and abandoned, owing to the great volume of objectionable gases evolved, probably accompanied by heavy losses of silver. Gutzkow’s original method of reducing sulphate of silver by a solution of proto-sulphate of iron is now used at the company’s works at Argentine, and 36,000 ozs. of dore (containing on an average 4 per cent, of gold and 95 per cent, of silver) are parted daily at a mean cost (in 1895) of 0.22 cent per oz., not including cost of superintendence and office expenses. The cost in 1892 at this works had been 0.35 cent per oz. The wages are from $2 to $2½ per day, and sulphuric acid costs one cent per pound.
