By modelling the basic flotation equipment from the early 1900’s you see that most can hobby prospectors can build or assemble basic froth flotation equipment using all the machines and concepts explained below and perform home-based flotation tests. Although the subject of testing for flotation has been well presented in T. J. Hoover’s book on ‘Concentrating Ores by Flotation, there is need of later information on this timely subject. Much testing has been done in laboratories not connected in any way with the Minerals Separation company, with which Mr. Hoover was formerly associated as metallurgical engineer, and there have been developed methods of investigation that may prove suggestive to many experimenters.
On that account we have compiled data on the subject of testing both from the literature available and from our own experience, as well as from what we have seen in other laboratories. This paper is designed to present the results of this compilation, with a critical discussion of the more important methods now in vogue.
On account of the empirical state of the art of flotation a great deal of testing is necessary before large-scale practice can be commenced on any ore; therefore a small laboratory-machine is necessary in which many tests involving many variables can be made in a short time. The machine must be so designed and so operated that a close approximation to the results possible with full-sized flotation machinery will be obtained. In a mill-plant it is a matter of some difficulty to control conditions through a wide range of such variables as temperature, acidity, quantity of oil, percentage of solids in pulp, fineness of grinding, etc., and as the proper treatment of a given ore can be ascertained only through testing it first, a critique of the testing methods in use is in order.
Many people have had the experience of reading the available literature on flotation-testing and of failing to get satisfactory results when the described testing was attempted. To actually witness some good test-work and learn thereby the appearance of froth, the exact manipulation of the machine and froth, goes far toward bringing the beginner to a point where he can test efficiently.
None of the literature mentions the fact that it is difficult to get a high percentage of extraction and a high grade of flotation concentrate at the same time. The beginner often strives after both of these things in a single test, whereas he should determine how each can be attained before he attempts to obtain both simultaneously. Furthermore, it is difficult to manipulate a small machine to give as good results as a large one, until after considerable practice. 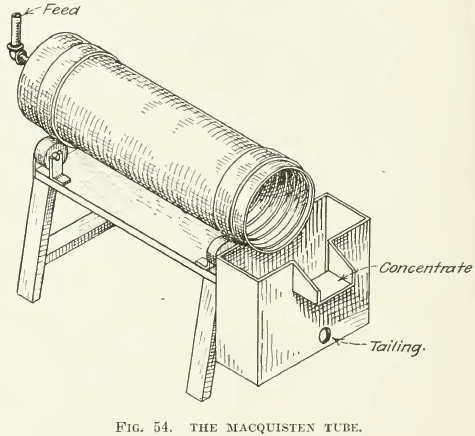 So the small machine is generally pessimistic, compared with the large one. It is practically essential for the beginner to weigh and assay all of his products in order to see if the extraction and the grade of concentrate are satisfactory, where an experienced manipulator can often tell by aid of past experience and the use of a glass or microscope whether he is getting good results or not.
So the small machine is generally pessimistic, compared with the large one. It is practically essential for the beginner to weigh and assay all of his products in order to see if the extraction and the grade of concentrate are satisfactory, where an experienced manipulator can often tell by aid of past experience and the use of a glass or microscope whether he is getting good results or not.
With these points in view, we shall describe first the satisfactory machines and their operation. Then we shall give a more general exposition on what variables to study and what points to observe.
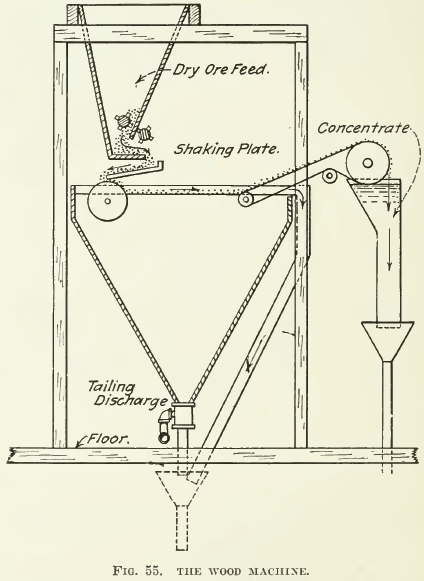
Flotation test-apparatus must necessarily be classified in the same way as large-scale machines, namely, as film-flotation machines, acid- flotation machines, and froth-machines of both pneumatic and mechanically agitated types. Film-flotation, as exemplified in the Macquisten and in the “Wood machines, does not seem to have the same wide application as does froth-flotation; hence little need be said about them.
FILM-FLOTATION
Macquisten tubes have such small capacity that a single tube is small enough for test-work on a few pounds of ore at a time (see Fig. 54). A small 4-ft. tube is known to give trustworthy results, although a longer one is more desirable. Testing with a Macquisten tube was done for several years in the laboratory of the General Engineering Co., of Salt Lake City, of which company J. M. Callow is president. Since Mr. Callow has begun the exploitation of his own pneumatic frothing-machine this work has been set aside.
The Wood machine can be built in miniature and for several years a small machine of the type sketched has been used in the plant of the “Wood ore-testing works at Denver. This small machine was about two feet long and one foot wide. The method of operation is the same as that of the full-sized machine. (See Fig. 55.)
As neither of these machines has been much used in practice, they are merely mentioned for the sake of completeness. Hoover has recommended a test on a vanning-plaque, so that the sulphides will float off onto the surface of the water, but we consider this test of practically no value. Hoover, however, acknowledges that it is merely a test illustrative of the film processes.
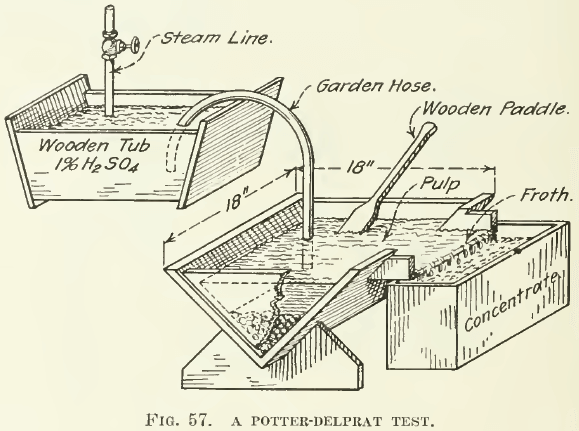
In testing ores for the Potter or the Delprat processes, Hoover’s text is again the source of information. An illustrative test-tube experiment is pictured in Fig. 56. Tubes containing 3% H2S04 or acid salt-cake solutions and a little sulphide ore are warmed nearly to the boiling temperature. Bubbles of C02 attach themselves to the sulphides, travel to the surface of the solution, discharge into the air, and drop the sulphides into the pocket on the under side of the tube, as shown in the annexed sketch. In another test a 200-c.c. beaker is used with 100 c.c. of 3% H2S04 and brought to nearly boiling temperature. The ore when introduced into this yields a froth composed of sulphides supported by bubbles of Co2. In case the ore is deficient in carbonate, an addition of as much as 3% of calcite or siderite is made. The froth is skimmed with a spoon as soon as it forms. We have noticed that a great deal of mineral is often lifted partly but never reaches the surface. Consequently extractions are low, although the grade of concentrate obtained is often very good. For practical purposes, however, the test is not of much value. A better test-machine is the small unit shown in Fig. 57. The acid should be allowed to run down through a section of garden- hose to within an inch of the surface of the ore and the ore should
be kept stirred with a wooden paddle so that the bubbles of C02 generated by the action of the acid can lift the sulphides out of the body of the pulp. The froth formed should be skimmed with the paddle as fast as made, then filtered, dried, weighed, and analyzed. Not many ores yield gracefully to this treatment and slimes give poor extractions. Fines and Wilfley-table middlings are better adapted, and the presence of siderite in the pulp is desirable, as it reacts slowly with dilute acid. From 1 to 3% H2S04 is best in testing and ½ to 1½% solutions on the large scale will give about the same results. The temperature of the pulp should be maintained at 70° C. by use of a steam jet. Five to ten pounds of ore per test is necessary. The extractions obtained are always lower than in full-sized units. While oil is not necessary in this process, it will greatly assist in the flotation, and the addition of a small amount is often of much assistance in test-work.
MECHANICAL FROTHING
MECHANICAL FROTHING as developed by the Minerals Separation company in England and Australia, and modified by many others, has been one of the most important methods of flotation. Therefore the laboratory machinery that has been developed is at as high a state of perfection as any such machinery now in use.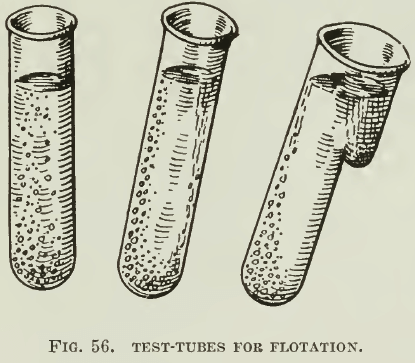
The Janney machine is probably the best designed machine for getting reliable quantitative results on a small quantity of ore. Photographs and sketches are appended (Fig. 58, 59, and 60). It can be seen that the agitation compartment is cylindrical in shape and that its top is surrounded by a froth-box, which slopes into a spitz- kasten, where the froth can be skimmed. The tailing sinks to a return-hole at the bottom, passing into the agitation-compartment again. To provide good agitation, four vertical baffles are attached to the wall of the agitation-compartment, against which the pulp is swirled by the two impellers. Lining the walls with expanded metal lathing or with a coarse-mesh iron screen adds to the thorough mixing that the pulp must receive. The two impellers are on a common shafting, which enters the machine through a stuffing-box in the bottom of the machine. The lower impeller with four vertical vanes is submerged; it agitates and emulsifies the pulp while the upper impeller, likewise with four vertical vanes, acts as a pump to lift the pulp and beat air into it. A pulley and belt connects the shafting with a variable-speed motor.
A dome-shaped lid is used on the machine. A small hole in the top of the dome allows the introduction of oil, acid, water, or other materials without the removal of the lid. The lid is so constructed that it can be turned upside-down with the dome
extending down into the froth-box, and in this position it can act as a funnel. The dome rests then on the top of the agitation- compartment and no froth can escape into the froth-box. This allows a period of agitation of the pulp before the dome-top is turned right-side up to allow aerated pulp to overflow into the froth- box and down into the spitzkasten, where the froth can be removed.
A discharge-plug at the bottom of the machine allows the flushing out of tailing after the test has been completed. So careful has been the design of this test-machine that even this discharge-plug is beveled to fit flush with the bottom of the machine and thus afford no dead space in which the solids might settle.
The spitzkasten is long and narrow, in order to permit a deep froth to be formed and to travel over as long a space as possible, before reaching the discharge. This tends to allow more of the entrained gangue to settle out of the mineral froth. The sides of the spitzkasten are of heavy plate-glass, each fastened to a metal frame by means of screws. The wrought-iron shaft projects through a brass stuffing-box and is supported by a ball-bearing beneath. All the other metal parts are of cast aluminum.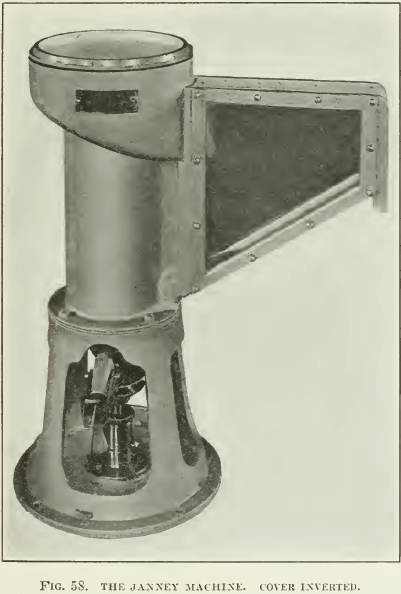
The small variable-speed motor may be of either D. C. or A. C. type. F. G. Janney recommends the use of a General Electric, shunt-wound, direct-current motor, for 230 volts, with a rated speed of 1700 r.p.m. and ¼ hp. The impeller-shaft is to be driven at 1900 r.p.m. maximum speed. For speed-control he recommends a General Electric direct-current field-rheostat, with an ampere capacity of 1.25 to 0.063 at 250 volts.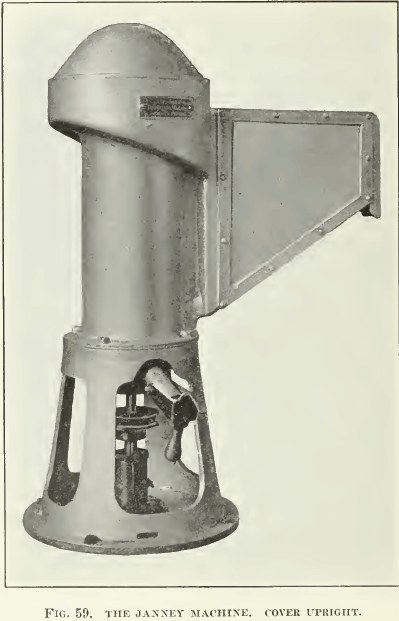
In our own laboratory it was desirable to use the ordinary city-lighting circuit of 110 volts, A. C. On that account we have found the following motor satisfactory: ¼-hp. General Electric repulsion induction motor, single-phase, 60-cycle, with full speed of 1780 and carrying 4.2 amperes at 110 volts, or 2.1 amperes at 220 volts, depending upon the voltage of the current supplied to the machine, either voltage being acceptable. Speed-control is obtained by the use of an ordinary field-rheostat in series with the motor. Such a motor has a speed varying with the load and with the voltage applied. As the load is practically a constant, the speed will depend upon the amount of resistance in series with the motor. As the majority of laboratories find a city alternating current more convenient to obtain, such a motor is recommended.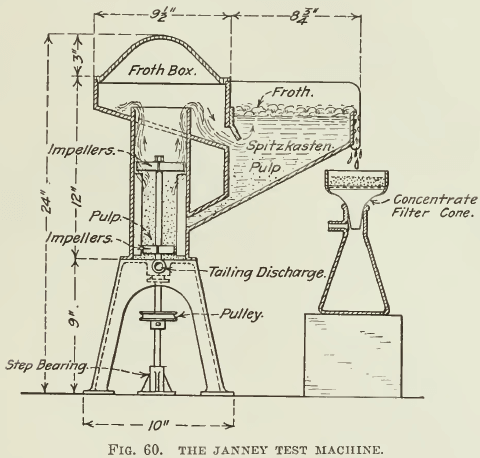
The operation of the machine is as follows: It is set up on a bench convenient to the sink and to running water. The motor is set up one foot to the rear with the switch and rheostat placed so that they can be easily reached while standing in front of the machine. A ¼-in. round-leather sewing-machine belt is used for drive. The bearings are well oiled, the stuffing-box is properly packed, and some attention should be given to it occasionally in order to see that it is kept screwed tight enough to avoid leakage.
Enough clear water is run into the machine to barely show in the spitzkasten and the motor is started at its lowest speed. A 500-gm. charge of ore ground to at least 48-mesh is added and the cover placed on the machine in its inverted position. (See F’ig. 58.) This is done to allow thorough mixing without circulation of the pulp. All or part of the oil and other reagents are now added and the motor brought up to full speed for 30 seconds. The speed is again lowered to the minimum and the cover is turned over into its upright position. (See Fig. 59.) The speed is then raised and water is added through the hole in the top of the lid until the froth in the spitzkasten is nearly at the overflow lip. The ultimate speed of the agitator will depend somewhat upon the character of this froth, as some oils will give a deep persistent froth, while other froths are thin and brittle and allow of more water being added to the machine, as well as more violent agitation in order to beat more air into the pulp. The froth may either be allowed to flow out of the spitzkasten of its own weight or skimmed with a small wooden paddle. It is a good idea to wet the glass sides of the ‘spitz’ with water while the froth is rising, so that none of the froth will stick to the glass.
The duration of the test is about five minutes with an ore that floats easily, while other ores will require a considerably longer time to allow the entrained gangue to settle out of the froth before it is discharged from the machine. In such cases it is best to hold back the froth until its appearance shows it to be fairly clean. Beginners are likely to dilute their froth with too much gangue. In a large-sized machine the froth can travel over from four to eight feet of spitzkasten before it is discharged, while in this test-machine it only has a travel of about 10 inches. Consequently, the small machine is liable to yield concentrate of too low a tenor. The same applies to most other machines for making tests on flotation.
The concentrate may be caught in a pan or on a filter. After the test the machine is brought back to low speed and the tailing- plug removed, so that the tailing can be caught in a pan or bucket, or run to waste.
If it is so desired, this rough concentrate can be put back into the machine and treated in the same way as the original sample, or the concentrates from several tests combined to give enough material for re-treatment. If this is done three products are made, namely:
- A ‘rougher’ tailing, to waste.
- A clean concentrate, for shipment.
- A ‘cleaner’ tailing or middling, which in actual practice is returned to the head machine.
When these conditions are observed results only slightly lower than those possible with a big machine can be obtained. A test can be run in from 5 to 30 minutes in such a machine with 500 grams of ore in anything from a 3:1 to a 5:1 pulp. The glass sides of the spitzkasten allow close observation of the condition of the froth, and this is a great advantage to the beginner. The small amount of ore necessary for a test is a matter of considerable convenience as fine grinding of the ore in the laboratory is often irksome. The aluminum casting is little corroded by either acid or alkaline electrolytes. The return of pulp from the ‘spitz’ to the agitating-
compartment allows the material to be treated until all mineral has been removed without stopping the machine, so that a single treatment yields a clean tailing. However, a second treatment of this ‘rougher-froth’ is sometimes necessary in order to get a high-grade concentrate. Clean tailings generally mean only medium-grade concentrates due to entrainment of gangue, in the removal of all the mineral.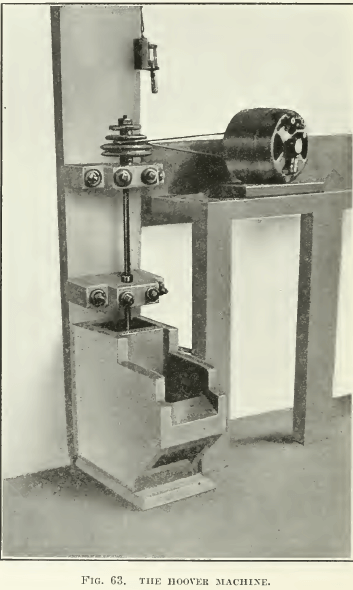
The stuffing-box in the bottom will probably leak if not watched. However, this driving of the impellers from below, instead of from above, leaves the top of the machine free for the operator and is more convenient in every way. This is of importance in a laboratory- machine, and will excuse the use of a stuffing-box. In large-scale machines a stuffing-box underneath would not be tolerated, and the drive should be from above. We would also suggest a sheet-lead construction as being more easily built. A ¼-inch sheet-lead is sufficiently rigid to stand up well, while it is ductile enough to be
A. Spitzkasten.
B. Agitation compartment.
C. Variable-speed motor.
D. Retaining bolts.
E. Impeller.
F. Concentrate discharge.
worked readily into the desired shape. The joints are easily burned, and it is acid-proof.
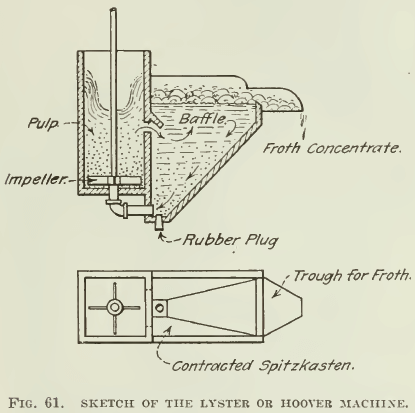
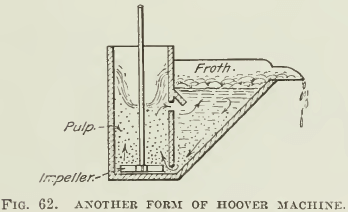
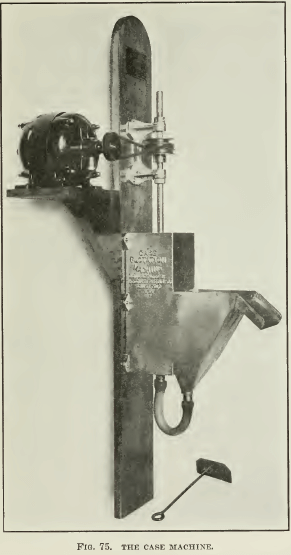
THE HOOVER MACHINE, so-called, was designed after a test- machine described in the second edition of Hoover’s book, being copied from one of Lyster’s patents, and has been much copied by people wishing to make flotation tests. An improvement over this construction was published by Ralph Smith recently (see Fig. 61), and a modified sketch of the same is shown in Fig. 62, while photographs of the machine used for a while in our laboratory are shown in Fig. 63 and 64. Either a variable-speed motor is belted to the pulley that drives the stirring mechanism, or a pair of cone- pulleys on a constant-speed motor is used. This construction has been popular because it can be made of wood, at small expense. The Janney machine will cost about $100, while the Hoover machine can be built for a small fraction of that amount. Mr. Hoover’s original drawing does not show the spitzkasten drawn to a point, as only the front side was beveled. Our sketch shows both sides beveled. This is desirable, as it eliminates space in which fine sand can settle, and tends to minimize the amount of pulp lying inactive in the spitzkasten. In the agitation-compartment the pulp is swirled into the corners, where it is well mixed with air; hence the baffles sketched in the Janney machine are unnecessary. One objection, however, is that unless the agitation-compartment is very tall the pulp being swirled into the corners has a tendency to splash out, and a lid similar to the one on the Janney machine is desirable. However, it is difficult to attach one because the stirrer- shafting is in the way. The operation of this machine is practically the same as that of the Janney, except that without glass sides on the spitzkasten it is hard to get as clean a froth. A charge of 1000 to 2000 grams is necessary in this machine.
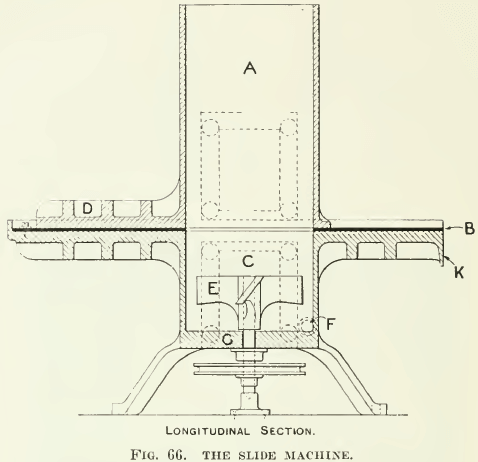
THE SLIDE MACHINE
As shown in Fig. 65 and 66, was designed by Hoover and perfected by many others. In recent practice it is motor-driven. A number of these machines were given by James M. Hyde to various universities in this country. Many people favor this apparatus for the reason that they have had little opportunity to use any other design. In this machine the agitator is driven from below through a stuffing-box, as in the Janney, with the consequent freedom of the top of the machine for the convenience of the operator. The top half of the machine is so constructed that it can be slid to one side, cutting off the froth formed in the agitation from the gangue, which is allowed to settle. The operation consists in agitating with oil and other reagents, then a period of quiet during which the froth collects at the top while the gangue sinks. Two windows in the side enable the observer to see when the gangue has subsided sufficiently to allow the top half to be slid along the rubber gasket, cutting off the froth from the remainder of the pulp. The time necessary for the settling of the gangue is sufficient for much of the gangue to separate from the froth, leaving only clean sulphides in the froth. This element of the machine has made it of some value in testing flotation oils, but in a weak froth
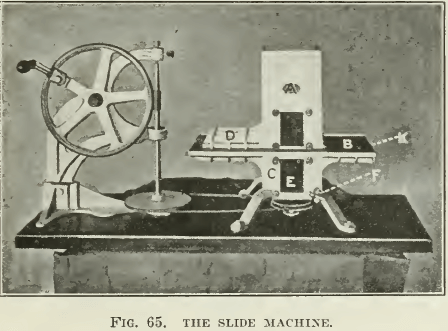
much of the sulphide mineral also settles out and is lost, so that the test results with this machine often show unnecessarily low extractions and a high grade of concentrate. On the other hand, when conditions are adjusted to give a froth persistent enough to hold all the sulphide mineral, considerable gangue is entrained in the stiff froth. Further, after skimming one froth we find it necessary to add more water and start the machine again to make more froth. It is hard to make the slide machine give a high extraction with only one agitation. The intermittent character of such work and the time necessary to wait while settling are disadvantages that make the Janney or the Hoover machines of greater utility, in our opinion. The parts are of cast aluminum with a rubber gasket between. A charge of 500 to 1000 grams of ore is used.
As regards the fineness of crushing in laboratory work, material ground as fine as 200-mesh will yield high extractions with much
A. Upper part of cell.
C. Lower part.
B. Rubber cushion.
D. Tail to prevent leakage of froth.
E. Agitator.
F. Hole for withdrawal of tailing.
greater case than coarser material. It is possible to get acceptable work in some cases with material as coarse as 40-mesh on condition that there is a considerable portion of the same material in the slime. For ordinary laboratory work a convenient size is 80-mesh, unless poor extractions are obtained.
SEPARATORY FUNNELS
During the past year an article on practice in Mexico mentioned the fact that much of the preliminary testing on the ore was done in separatory funnels, in which the charges of pulp, oil, etc., were shaken, after which the cock at the bottom of the funnel was opened and the tailing run into a second separatory funnel for further flotation tests, the cock being closed in time to catch the froth. The versatility of experiment permissible with the use of such apparatus (Fig. 67) is commendable. Obviously, this arrangement is open to the same objections as is the slide machine except that separatory funnels are simple and inexpensive.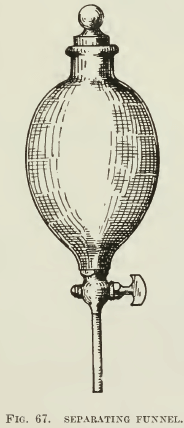
Elmore Machine. As far as we know, no small test-machine for the Elmore process has come into common use on account of the fact that the pulp must be lifted through a tube corresponding in length to the column of water equivalent to barometric pressure. This makes an awkward laboratory machine. Mr. Hoover (2nd edition, page 98), describes “illustrative” experiments with the pulp in a bottle connected with a water-pump for producing a vacuum, but no quantitative method of this kind has been developed.
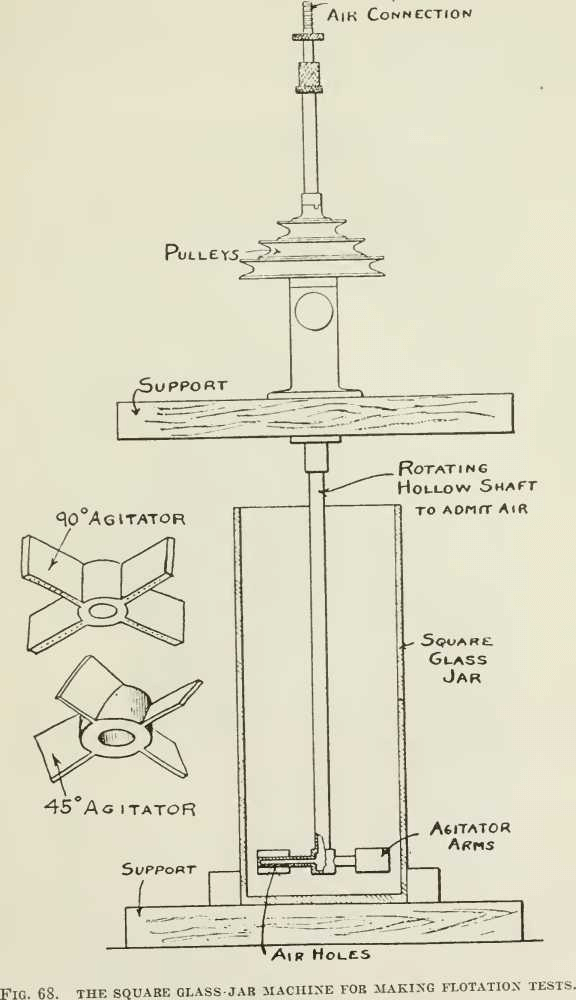
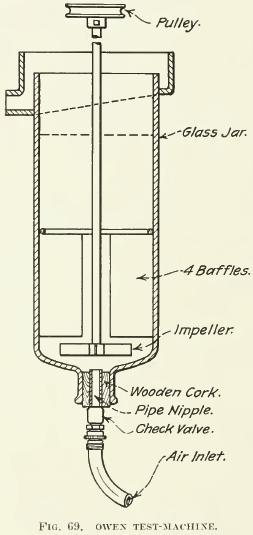
Other miscellaneous frothing tests are in the literature but most of them are merely “illustrative.” Putting a charge into a soda- water siphon, pumping in air to dissolve the water, and then releasing the charge into a beaker gives nice-looking froth. In some of the lawsuits square glass candy jars (Fig. 68) with a motor-driven impeller have been used to show flotation phenomena in court. In a recent U. S. Patent (No. 1.155.836) taken out by T. M. Owen, one of the engineers of the Minerals Separation company, is a sketch of a simple test-machine made of an ordinary 2½-litre acid-bottle. (See Fig. 69.) This corresponds to the sub-aeration type of machine and is recommended by Mr. Owen for test-work when such a type of machine seems necessary, as in differential flotation. Air is led into the pulp through the stopper in the bottom and beaten into the pulp by the impeller. The four large baffles above the impeller prevent the swirling of the pulp from rising through them, so that there is a quiet zone in the top of the machine where the froth can collect. One great beauty of such a machine is that any froth formed will rise immediately to the discharge. However, we believe that the Janney and Hoover machines are the most useful of the mechanically-agitated type.
PNEUMATIC FLOTATION
Among the different pneumatic machines, as far as we are acquainted, the Callow test-machine is the only one of laboratory size that has been much developed. It is merely the commercial Callow machine reduced in size (see Fig. 70, 71, and 72). Later development in the laboratory of the General Engineering Co., in Salt Lake City, has resulted in the reproduction of the whole plant in miniature (as shown in Fig. 72), with a Pachuca mixer, a roughing-cell, cleaning-cell, vacuum-filter, and sand-pump to return middling to the Pachuca mixer. As seen in the drawing, the pulp is mixed well in a Pachuca tank of small size, overflowing into the rougher flotation-cell. The tailing from this rougher goes to a sand- pump and is returned to the Pachuca. The froth is treated in a
second and smaller pneumatic-flotation unit, giving a concentrate that overflows into an ordinary laboratory vacuum-filter actuated by a water or aspirating pump. The tailing from the ‘cleaner-cell’ consists of a middling that likewise flows to the sand-pump and back to the Pachuca.
A novice will have no small difficulty in operating such an installation, as there are a number of things to be kept in operation at the same time. The mixture of ore, water, oil, and any other reagents is fed either into the suction of the sand-pump or into the top of the Pachuca after air has been started into the various machines. The overflow from the Pachuca into the rougher-cell accumulates until a nice froth is coming up and nearly overflowing. Then the tailing-discharge valve on the rougher is gradually opened and froth allowed to overflow from the cell into the ‘cleaner’-cell. It is best to get most of the charge circulating before much concentrate-froth is allowed to overflow, the overflow of froth being controlled by the main air-valves leading to each unit. After the valves into the individual wind-boxes beneath the machine have been once adjusted they should never be disturbed, and all control of air supplied should be at the valves in the main pipes.
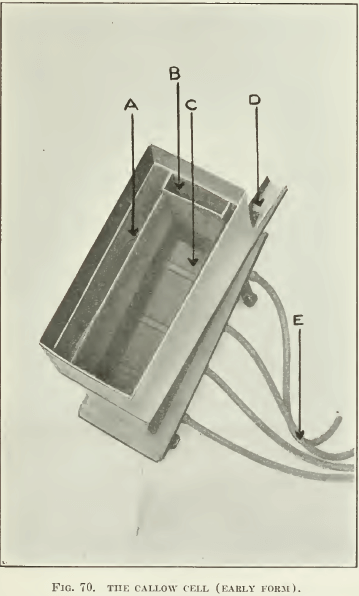
A. Froth-overflow launders.
B. Pulp-feed to air-blankets.
C. Air-atomizing blanket.
D. Concentrate discharge.
E. Compressed-air feed to wind-boxes.
When everything is going well, the air-pressure in the cleaner can be increased until concentrate-froth is overflowing into the vacuum-filter. A wooden paddle to stir any settled material in the flotation cells is of value, as well as a small jet of water from a rubber hose for washing concentrate along the froth-launders and for beating down froth when occasional too-violent rushes of froth from the cells take place. After a test is complete the pulp should be drained completely from all parts of the machine while the air is still blowing, so that
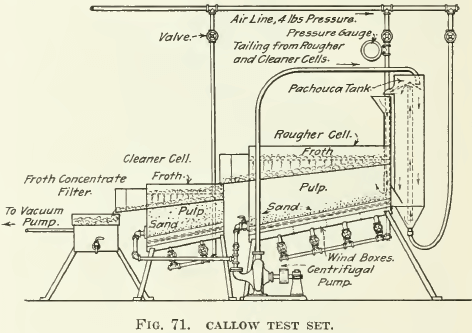
solids will not settle in passages or clog the canvas blanket in the cells. Only practice will allow anyone to get reliable results with this machine. A watch-glass for catching and panning occasional samples of froth is another necessary auxiliary to this equipment. The cost of installing such a set of apparatus is from $100 to $150. At least 1000 grams of ore is required for a test and about 30 minutes to 1 hour is spent. It can be seen that nothing but a finished concentrate and a tailing are obtained or a middling product may be left in the cleaner cell. This middling may be assayed as such and calculated into the concentrate and tailing or its sulphides may be panned out and added to the concentrate. The machine is said to give results closely paralleling those obtained with larger-scale apparatus. A source of supply of compressed air at 3 to 5 lb. per sq. in. is necessary and the main valves on the air-pipe leading to each machine should be some type of needle-valve in order to ensure exact control.
In testing practice, the air-lift type of middling-return has been found more satisfactory than the centrifugal pump shown in Fig. 71.
LABORATORY MANIPULATIONS
Turning from the description of the machines used to the operations on the ore before and after the flotation operation, we have in general the problems of crushing the ore and of drying the froth-concentrate.
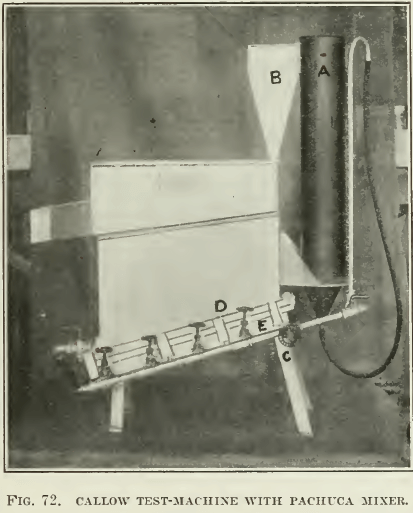
A. Pachuca mixer.
B. Pulp-feed to air-blanket.
C. Needle-valve air-control.
D. Blanket-clamps for quickly removing blanket for cleaning.
E. Wind-boxes.
As a rule laboratory machinery for the pulverization of ore is of the dry-grinding type, with the exception of small ball-mills that can crush from 1 to 100 lb. charges in the wet. Consequently, most people start with weighed charges of finely-ground dry ore, a known quantity of water, of oil, and of acid or alkali. Our experience has been that most dry-ground ore must be treated in an acidified pulp to get good flotation. Doubtless the surfaces of sulphide particles become somewhat oxidized in, or shortly after, dry grinding and the function of the acid would be to clean the slightly oxidized surfaces. Wet grinding usually does not call for so much acid. In nearly all laboratory work finer grinding than is used in practice seems to be necessary. This may possibly be due to the smaller amounts of froth that are formed. Such small quantities of froth cannot form layers as deep as those made in the large machines. If a big particle of sulphide can be entrained with a number of smaller particles, it can be floated, but with a thin froth the chance of such entrainment would seem to be less. Some experimenters have informed us that they were able to float even as large as 30-mesh material, but our own experience is that 60-mesh material is often hard to float with any chance of getting a high extraction, while the operation is performed with much more ease and expedition when the ore is crushed somewhat finer.
Wet grinding is more desirable, as it parallels conditions in practice, where most of the finer grinding of ore is in Chilean, tube, and other mills. However, wet grinding is harder to manipulate in a small laboratory and requires more time. The dry weight of the feed to the flotation machine must be known; hence a weighed charge of dry ore crushed to about 10-mesh can be introduced into a porcelain or iron pebble-mill for grinding and ground for the length of time found necessary to reduce the pulp to sufficient fineness—15 minutes to 24 hours. The charge can then be poured and washed through a coarse screen (to retain the pebbles) into a bucket and thence into the flotation machine. The oxidation of sulphide surfaces is thus avoided, but separate grinding of each charge, in order to know its exact weight, is rather tedious and requires a number of small mills if many tests are being run, on account of slow speed in grinding. A mill with iron balls rather than pebbles is of greater service. It is possible to introduce the flotation-oil before grinding, to be sure that it will be thoroughly mixed. For thick viscous oils this is highly beneficial, as a ball-mill gives about the best conditions for agitation and mixing. Usually 1 to 2 lb. charges are used and a small laboratory mill of the Abbe type serves well, although a good mill can be made with a 10-in. length of 8-in. iron pipe and two heavy iron caps for the same.
Practice in our laboratory has been standardized to a miniature gyratory crushing to 10-mesh, splitting into weighed samples kept in paper bags and reduced to smaller size by either wet or dry grinding as occasion demands.
A short-stemmed tin funnel about 6 inches in diameter with a one-inch opening is found to be about the most convenient means of pouring a charge of ore into a laboratory flotation-machine.

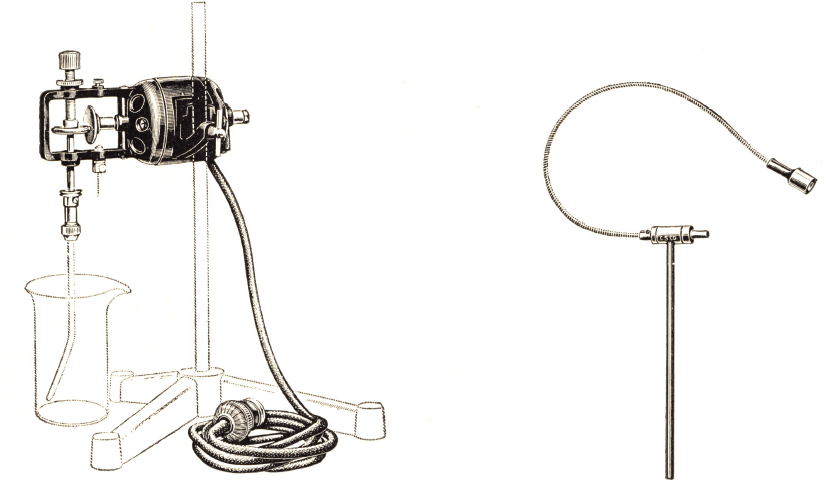
The measuring and testing of flotation-oils in the laboratory has been very inexact in many instances witnessed by us. It is common practice to count the number of drops of oil falling from a small piece of glass tubing. We are using a Mohr pipette of 1 c.c. total capacity for measurement of the amount of oil used in each test. Such a pipette is shown in full size in Fig. 73. It will be seen that this pipette allows measurements of the oil to the nearest 0.01 c.c., which is as close as will ever be desired. If the density of the oil is known, the volume as measured by this method is quickly converted into the weight of oil used.
The testing of oil samples for flotative power is a matter that needs standardizing. It is desirable to classify oils according to flotative power, but just how to do this is not exactly clear. A unit of ‘flotativeness’ might be established and each oil referred to that unit in terms of percentage. But it has to be remembered that the best oil for one ore may not prove to be the best oil for another, although two such series of oils might roughly parallel each other. For any given ore, it would be permissible to make such a measurement on a series of oils and group them according to some definite standard. A standard oil might be chosen and the value of a second oil expressed in percentages of the flotative power of the first as determined by using equal quantities of the two oils in tests on an ore under identical conditions. This test could not be fair for the reason that different amounts of two different oils are necessary to accomplish the same results. Further, the conditions of acidity or alkalinity might favor one oil and handicap another. If we measured the amount of oil necessary to give a fixed percentage of extraction the first of the above objections would be satisfied, but conditions of acidity or alkalinity could make the test unfair for some oils. Hence the dilemma as to a standardized test of a flotation-oil.
No single test could definitely place an oil in any scheme of classification and nothing can be done but run a series of tests using varying amounts of the oil to be tested and with varying acidity or alkalinity. The temperature of the pulp must be kept constant although it has a minor effect.
Coutts gives about the only directions on oil testing that are to be found in the literature of the subject. He states rightly that the first thing to do with an oil is to measure its density, for future calculations, as it will be measured by volume in the laboratory and must later be reduced to weights. He recommends the use of a burette for measuring the oil, but we favor the Mohr pipette mentioned above. He chooses a standard ore on which all tests are to be run and classifies three different kinds of standard tests: (1) for mixed sulphides, (2) differential separation, and (3) flotation of copper and iron sulphides. He states that oils high in phlanderene have proved best for differential separation of zinc-lead sulphide ores. “While this is helpful, he does not state just how the oils are to be classified after the tests have been made.
Much work with oils is needed in order to determine if there are any definite constituents in oils that give them flotation power. Research is also needed in the preparation of oils from the wood, coal, and mineral oils in such a manner that they will have maximum efficiency in flotation. Work on this subject has been initiated in our own laboratory and it is known that several of the larger companies have employed oil chemists to look into such problems. We understand that most excellent work is being done on methods of modifying and reconstructing oils that can be had cheaply. By this we mean more than mere mixing of a good flotation oil with a cheaper non-selective oil. Sulphonating the oils, dissolving them in acids, dissolving modifying substances in the oils, etc., are some of the ideas being tested with varying success. It is 0n account of all this oil testing that considerable progress has been made in flotation during the past year, so that now most of the larger companies are using cheaper oils than a year ago.
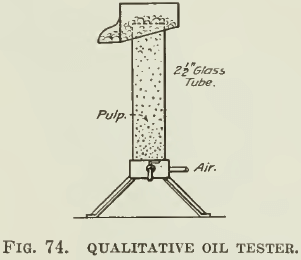
When starting to work with a new ore, there is needed a rapid qualitative method of choosing an oil that seems well adapted to the flotation of the ore in question. Such a scheme is in use in the laboratory of the General Engineering Company at Salt Lake City. Their qualitative tester is designed to test oils for use in the Callow pneumatic flotation cell and consists of a glass tube of about two inches diameter and two feet long. (Fig. 74.) This can be set on end and closed at the bottom with a one-hole rubber stopper through which passes a glass tube into a small canvas bag. The small bubbles of air coming through the canvas are similar to those used in large-scale machines and can be observed through the glass walls of the tube. With some pulp in the tube, oils, acids, salts, etc., may be added in very short tests until the proper appearance is obtained. An overflow lip is provided in case it is desired to examine the mineral in the froth. A slight adjustment of the air will provide an ample overflow of froth.
DISPOSAL OF THE FROTH
The handling of the flotation froth in the laboratory finds difficulties which are reflected in practice.
It is often very slow to settle and filters with difficulty. A vacuum- filter, connected with a laboratory aspirating pump, is a very convenient method of getting the concentrate out of the froth. A large porcelain Buechner funnel fitted into a filtering flask, as shown in Fig. 60, is used at present in our laboratory. A copper vacuum- filter of much the same type, provided with a porous false bottom of acid-proof wire cloth, resting on a punched plate, is shown in Fig. 71 of the Callow test set. Filter-papers can be laid over the bottom of either of these funnels to collect the concentrates, and the vacuum beneath sucks out the water and oil of the froth. Such a filter can be placed under the froth-discharge of a flotation machine so that a fairly dry cake of concentrate is ready for further drying at the end of the flotation test. By loosening the outer rim of the filter-paper and then turning the funnel upside down over a pan, the filter-paper with the concentrate can be dropped into the drying-pan by gently blowing into the stem of the funnel. This is set aside in a warm place to dry and later weighed against a filter-paper tare.
If it is desired, the froth can be collected in a glass beaker or other vessel and allowed to stand over-night. A layer of clear water can then be siphoned off and the thick pulp remaining filtered or dried direct. In some laboratories the froth is dumped onto a shallow pan on a hot plate and the water evaporated. Occasionally such a sample of froth will be left too long, and will be ignited and roasted. We once used a numbered set of shallow pans for such evaporations but prefer filtering before drying the precipitate. A numbered tag is now put in each pan along with the cake.
The products coming from the flotation machine should be watched closely and occasionally panned or examined with the microscope to see what kind of work is being done. This is fairly easy to determine as the sulphides are most of them distinguished easily from the gangue under the microscope, and likewise gangue particles in the froth-concentrate can often be distinguished. A microscope is a most useful adjunct in a flotation laboratory or mill.
GENERAL CONSIDERATIONS
We have mentioned at various places the relation of the laboratory tests to the large-scale operations and now repeat that in almost every instance the laboratory results are somewhat pessimistic as compared to large-scale work. The reasons are made apparent by the smallness of the machine and the shallower layer of froth often formed under these conditions. Moreover, laboratory operations seem to call for greater amounts of oil, acid, etc., than do the large-scale operations.
Only one of the above machines is adapted to ‘roughing’ and ‘cleaning’ operations in a single test. Present-day practice tends toward re-treatment of at least part of the froth in order to make cleaner and higher-grade concentrates. Consequently, it may be desirable to collect enough froth from a series of tests to be re-treated in a ‘cleaning’ test. Of course, this is provided for in the Callow test set, where only ‘cleaned’ concentrate is discharged from the machine. It is further found desirable to weigh and analyze some of the successive fractions of the froth being discharged from a flotation machine, as the tailing becomes leaner, and determine at what point it may be desirable to re-treat such froth.
Many reports of flotation test-work with mechanical-agitation machines give the speed of the rotation of the agitating-blades. We have found that it was possible to get much the same work done with quite a variation of speeds, the only effect being to lengthen or shorten the time of treatment. We feel that the importance of this matter has been much exaggerated. Some means of speed- control is necessary and the speed can be adjusted in each case until
the froth presents the proper appearance as to depth, size of bubbles, color, etc. Speeding toward the end of a test in order to give a deeper froth with a faint line of concentrate on the very top is often advisable. We recommend adjusting the speed in each test to suit the other conditions, rather than running a series of tests with different speeds. Only in the slide machine, where operation of the impeller must be suspended in order to allow froth to collect, is the speed of much importance. Here we recommend agitation for a definite length of time, and then a period of settling. The effect of variation of speed during a definite length of time may be a considerable variation in the amount of froth collected during the quiet period. Hence we are prejudiced against the use of the slide machine except for oil-testing.
When a good set of conditions has been found for the flotation treatment of an ore, it is best to recover the water from each test to see what effect a closed circuit of the mill-water will have. Some oil and chemicals are thus recovered, cutting down the amounts necessary for operation. In fact, a carboy or two of the water to be used in the large mill should be used to make certain that no deleterious contamination will ensue from this source. Under these conditions filtration of the concentrate and tailing for recovery of the water is necessary. Such conditions are provided for in the Callow apparatus, above described, and can be applied easily to any of the other machines.
Oil samples for test purposes can be obtained from the various wood-distilling companies now advertising in the technical press, from gas companies and from petroleum-refining companies.
In attacking refractory ores, there are a number of ingenious things that can be done to the pulp both in and out of the machine. The trouble may be due to deleterious substances, which sometimes can be washed out, rendered harmless by boiling, or by acidifying, or by making alkaline with lime before entering the machine. Occasionally, the ore will not work well under ordinary conditions but will yield beautifully after finer grinding. Sometimes extra reagents are necessary, such as powdered charcoal, modified oils, argol. soap, calcium sulphate, alum, etc. A rational method of devising the proper tests in such cases must be based on some theory of flotation. Colloid chemistry is a branch of knowledge that we believe to be very necessary for such work, as it has facilitated a more intelligent control of our tests and has given wonderful results in a number of instances.
Finally, it is well to be prodigal in the amount of analytical work connected with flotation testing in order to discover interesting differences in gangue-constituents carried into the concentrate, as well as to find the best conditions for leaving out some gangue constituent that is less desirable than the rest. If an experimenter does his own analytical work he can be expected to spend three-fourths of his time analyzing what has been done during the other fourth.
Summarizing the most important points to be tested on a given ore with any given flotation machine, we have:
- Method of grinding.
- Fineness of grinding.
- Kind of frothing agent used.
- Amount of frothing agent.
- Acidity or alkalinity.
- Temperature.
- Necessity of preliminary agitation.
- Effect of addition-agents in flocculating gangue-slime.
It can be seen that there may be a certain best combination of the above variables that will be entirely missed if a great many tests are not carried out; hence the desirability of doing the testing in a small laboratory-machine where many trials can be made in a short time.
After the best conditions have seemingly been established, they should be further tried in a larger-sized machine before they are incorporated into the general practice of a mill. The test-work on this scale need hardly be described, as, for the most part, it is a question of translation of laboratory results into large-scale operation.
https://archive.org/stream/flotationproces00unkngoog#page/n284/mode/2up
