Many precious metal ores contain copper minerals in various amounts. These copper minerals dissolve in cyanide solutions to a greater or lesser degree depending on the particular copper mineral or minerals present, their fineness, and the dissolving effect of the cyanide solutions. In the process of dissolution the copper combines with, or as it is termed, consumes cyanide and, if the amount thus consumed is appreciable, it might be a bar to the economical treatment of a precious metal ore by cyanidation. Mill solutions containing as much as 0.05% copper are not unusual.
Copper Cyanogen Complexes
Copper combines with cyanide to form a variety of complexes the preparation and properties of which are given briefly below:
CUPRIC CYANIDE, Cu(CN)2: Prepared by adding an alkaline cyanide to a water solution of a cupric salt in stoichiometric proportion:
CuSO4 + 2 NaCN = Cu(CN)2 + Na2SO4
Cupric cyanide, when precipitated thus, is greenish yellow in color. On standing for a short time, or on heating, the precipitate changes to white flocculent cuprous cyanide. This is due to the decomposition of the unstable cupric cyanide into cuprous cyanide and cyanogen:
2 Cu(CN)2 = 2 CuCN + (CN)2
In this respect it resembles cupric iodide which breaks down into cuprous iodide and free iodine.
Due to this instability of cupric compounds, it is unlikely that cupric cyanogen complexes exist for any appreciable length of time in mill cyanide solutions.
CUPROUS CYANIDE CuCN: Prepared by adding the required amount of an alkaline cyanide to a solution of a cuprous salt in water:
CuCl + NaCN = CuCN + NaCl
This complex is white, insoluble in water, insoluble in dilute acids and only slightly soluble in caustic alkalies. In the latter respect it varies from Zn(CN)2 which dissolves in caustic alkali to form zincate and double cyanide. It dissolves in strong acids thus:
CuCN + HCl = CuCl + HCN
A suspension of cuprous cyanide in water reacts with hydrogen sulphide forming cuprous sulphide and hydrocyanic acid:
2 CuCN + H.S = 2 HCN + Cu2S
This is probably only a surface reaction; as soon as the particles of cuprous cyanide are coated with cuprous sulphide further reaction ceases.
Cuprous cyanide is soluble in ammonia forming cuproaminonium cyanogen complexes of variable composition.
Cuprous cyanide reacts with ferric salts in the cold to form ferrous salts, cuprous salts and cyanogen:
2 FeCl2 + 2 CuCN =
2 FeCl2 + 2 CuCl + (CN)2
Cuprous cyanide dissolves in alkaline cyanide solutions to form a series of double cyanides, the composition depending upon the amount of alkaline cyanide present. The reactions may be expressed as follows:
CuCN + xNaCN = NaxCu(CN)x + i
where x = 1, 2 or 3

DOUBLE COPPER CYANIDES NaCu(CN)2, Na2Cu(CN)3, Na2Cu(CN)4: These complexes are formed by the action of NaCN on CuCN in the proportions indicated by the formula of the complex.
The complex NaCu(CN)2 is only slightly soluble in water, tending to break down into CuCN which is precipitated, and a higher complex in the series:
2NaCu(CN)2 = CuCN 4- Na2Cu(CN)3
The other complexes are readily soluble in water. All of these complexes are decomposed by dilute acids, precipitating CuCN and evolving HCN:
Na2Cu(CN)3 + 2HCl =
CuCN + 2 NaCl + 2 HCN
and in strong acids all of the cyanogen is converted to HCN:
Na2Cu(CN)3 + 3HCl =
CuCl + 2 NaCl + 3 HCN
A solution of sulphur dioxide in water reacts with sodium cuprocyanides in the same manner as a dilute acid, generating HCN and precipitating CuCN. An alkaline sulphide does not precipitate copper sulphide from sodium cuprocyanide solutions.
CUPRIC THIOCYANATE Cu(CNS)2: Is formed as a black precipitate when a soluble thiocyanate is added to a water solution of a cupric salt:
CuSO4 + 2 NaCNS = Cu(CNS)2 + Na2SO4
It is black, insoluble in water but soluble in ammonia. On standing, it decomposes to cuprous thiocyanate.
CUPROUS THIOCYANATE CuCNS: Prepared by adding a soluble thiocyanate to a water solution of a cuprous salt:
CuCl + NaCNS = CuCNS + NaCl
It also may be prepared by adding sodium thiocyanate to a cupric salt in the presence of a reducing agent such as sulphurous acid. Cuprous thiocyanate is a white salt insoluble in water but soluble in ammonia and alkaline cyanide solutions. It is decomposed by caustic alkalies forming yellow cuprous hydroxide. Strong solutions of sodium or potassium thiocyanate dissolve cuprous thiocyanate but it is precipitated again on dilution with water.
When cuprous thiocyanate is dissolved in cyanide solutions, a cyanide researcher did consider that it exists in solution as such. It has also been considered as existing in solution as a complex of the type CuCNS.3 NaCN. It is equally probable, however, that the cuprous thiocyanate reacts with sodium cyanide to form a sodium cuprocyanide and sodium thiocyanate:
CuCNS + 3 NaCN = Na2Cu(CN)3 + NaCNS
Ammonium Copper Cyanogen Complexes
It is well known that ammonia reacts with cuprous and cupric salts to form a large number of compounds. Their empirical formula show that combination has taken place between the particular copper salt and a varying number of molecules of ammonia. They are usually described as additive compounds. The ammoniacal cuprous compounds are colorless when pure but oxidize readily to form the corresponding ammoniacal cupric salts which usually have a deep blue color.
When aqueous ammonia is added to a solution of cupric sulphate, the blue complex formed has the formula Cu(0H)2 • 4 NH3 This is usually considered to be copper ammonium hydroxide,
[Cu(NH3)4] (OH)2, where the copper and ammonium are combined forming a divalent, complex cation, [Cu(NH3)4]++ Under some conditions, this cation can be formed in cyanide solutions containing copper.
Cuprous cyanide dissolves in ammonium cyanide to form ammonium cuprocyanide, NH4Cu(CN)2. Out of contact with air, this complex crystallizes in colorless crystals which are sparingly soluble in water; it readily gives off ammonium cyanide when heated leaving cuprous cyanide. When ammonium cuprocyanide is exposed to the air, oxygen is absorbed, and the colorless solution turns blue.
When evaporated in the presence of excess ammonia, green crystals of ammoniacal cuprosocupric cyanide, 2 CuCN•Cu(CN)2 • 4 NH3, are formed. The formula may be written 2 CuCN • [Cu(NH3)4] (CN) 2, indicating the complex cuprammonium ion. This complex is soluble in ammonia, but insoluble in water, and is unaltered by the air.
In its reaction to ammonia, cuprous cyanide is thus analagous to the copper halides. For example, in the case of cuprous chloride, hydrated cuprosic tetrammino chloride, CUC1*CUC12*4 NH3•H2O, is known.
When ammonia gas is passed into a solution of cuprous cyanide in ammonia, during crystallization, ammoniacal cuprosocupric cyanide containing 6 or more molecules of NH3 is formed. The crystals are deep blue in color and on exposure to the air lose ammonia passing into the insoluble green complex containing four NH3 molecules.
There may be many such cuprammonium cyanides containing CuCN, Cu(CN)2, and NH3 in various molecular proportions. Many of these complexes are said to be good solvents for gold in the absence of oxygen.
Solubility of Copper Minerals in Cyanide Solutions: Researchers have determined the solubilities of various copper minerals and metallic copper in cyanide solutions. They prepared synthetic ores from specimen copper minerals and sea sand. Each mineral, including the sea sand, was ground to minus 100 mesh. In the case of metallic copper, clean C. P. copper foil 0.002 inch thick was used.
The experiments were made under favorable conditions for cyanidation, which included continuous agitation in alkaline cyanide solution in the presence of excess air. The time of treatment was 24 hours and the ratio of solution to ore was 10 to 1. The cyanide strength of the solutions at the beginning of the tests was 0.10 percent NaCN. Tests were run at 23 °C. and 45°C. for each mineral. In these tests the copper in the various synthetic ores varied from 0.183% Cu to 0.267% Cu. The results of these tests showing the approximate relative solubilities of copper minerals in cyanide solutions are shown in Table 2.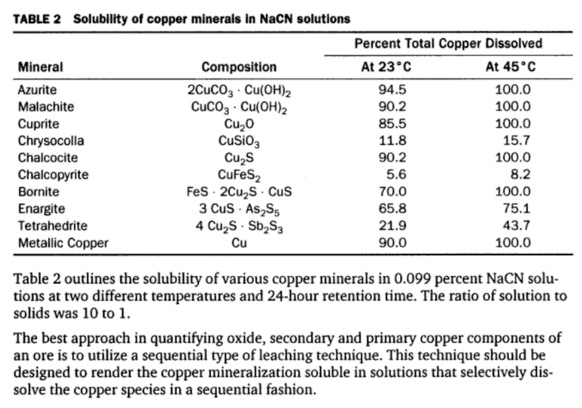
From these results it is apparent that azurite, chalcocite, cuprite, malachite and finely divided metallic copper are readily and practically completely soluble under the usual conditions for cyanidation. Bornite is largely soluble under the same conditions while enargite and tetrahedrite are sufficiently soluble to cause excessive cyanide loss and also to cause fouling of solutions with arsenic and antimony. Chrysocolla and chalcopyrite are the least soluble of the copper minerals. The rate of dissolution of all copper minerals decreases with a decrease in the temperature of the cyanide solution.
