Gold ore associated with Telluride is hardly soluble in ordinary cyanide solutions and special treatment is necessary for its extraction. There are two methods in use in such cases.
Roasting
The ore is ground dry to about 30 mesh and roasted; it is then ground to a slime in water or cyanide solution, usually in grinding pans, utilizing the opportunity simultaneously to amalgamate any gold that may be amenable, and finally treated by agitation in cyanide solution in the ordinary way.
Treating Raw with Bromocyanide
This process was used at the Deloro mine, Ontario, Canada, on a mispickel ore, and was developed in Australia by Dr. Diehl for the treatment of the sulpho-telluride ores of the Kalgurli district. The process essentially consists of grinding exceedingly fine, and then agitating with cyanide solution to which bromide of cyanogen is added at intervals. In some mills preliminary amalgamation and concentration are in use, the concentrate being roasted and then cyanided. During bromocyanide treatment the protective alkalinity is kept at the lowest possible point owing to the instability of the reagent, lime sufficient for settlement being added after the treatment is finished.
Julian and Smart state that at Kalgoorli when dealing with slimes assaying from 1 to 3 oz. of gold a wash of cyanide solution containing about 4.5 lb. of KCN to the ton of ore is first given, and after an hour or two’s agitation cyanogen bromide is added at the rate of about 1 lb. BrCN per ton of ore, and agitation continued for twenty-four hours. The quantities of free cyanide and bromocyanide are varied according to the assay value of the ore, any additional bromocyanide needed being added at intervals of several hours. Shortly before agitation is finished sufficient lime is added for the settlement of the slime.
The usual method of making the reagent for laboratory use is to add a strong solution of cyanide to bromine (and not vice versa), until the brown color is just discharged.
KCN + Br2 = KBr + BrCN
The quantity of BrCN may be determined in a working cyanide solution by acidifying with hydrochloric acid, adding excess of potassium iodide, and titrating the liberated iodine with decinormal sodium thiosulphate. Clennell gives the following reactions:
BrCN + HCl = HCN + ClBr
ClBr + 2KI = KCl + KBr + I2
1 cc N/10 thiosulphate = 0.00529 grm. BrCN.
The bromocyanogen is added to the cyanide solution to be used for extraction purposes in the proportion of about 1 of BrCN by weight to 4 of KCN.
Since BrCN is rapidly decomposed by alkali it is important that free alkali should be almost entirely absent during the treatment, the lime necessary for settlement being added at its conclusion. The reaction is usually illustrated thus:
BrCN + 2KOH = KBr + KCNO + H2O
The following is an extract from a paper by E. W. Nardin, of the Hannan’s Star Mill, Kalgoorli, published in the Mining and Scientific Press of October 24th, 1908.
“The bromo-cyanide solution is made according to the following equation:
(1) 2KBr + KBrO3 + 3KCN + 3H2SO4 = 3BrCN + 3K2SO4 + 3H2O
Its action in the treatment vat is supposed to be as follows:
(2) BrCN + 3KCN + 2Au = 2KAu (CN)2 + KBr.
“The first two quantities in equation 1 are contained in the mixed salts supplied by the London-Hamburg Co., having about 40 to 44% Br as KBr, and 20 to 22 % Br as KBrO3; the proportion of Br as bromide being about twice that of Br as bromate. A 30-lb. charge is usually made up, and for this the following weights are taken:

The KCN is 93%, and the H2SO4 63% (chamber acid) strength. The solution is made in a closed wooden vessel, stirred by rotating arms, holding about 200 gal. In making up a charge, a portion of the water and all the H2SO4 are first mixed, and allowed to cool to normal temperature. The KCN, which is dissolved in a separate vessel in sufficient water to fill the mixing vessel, is then run in, and at the same time the proper weight of ‘mixed salts’ is gradually added. The whole is then agitated for 6 hours before being used, and in a closed vessel it will retain its strength for some days. The cost of a 30-lb. charge of BrCN is about $21.88, made up as follows: 50 lb. H2SO4 at 4 c., 20 lb. KCN at 17 c., 36.8 lb. salts at 44 c. From each charge mixed, a dip-sample is taken and tested with a standard Na2S2O3 solution, using potassium iodide as an indicator.
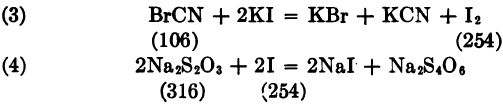
Thus BrCN corresponds to 2Na2S2O3.
“The standard solution is made so that 1 cc corresponds to 0.02 gm. BrCN, and for this about 93.6 gm. of ordinary photographic crystals, Na2S2O3 5H2O, are dissolved in one litre of water.”
“A solution of copper sulphate is used for standardizing the above.
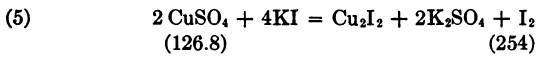
126.8 Cu liberates 254 I, so that it corresponds to 106 BrCN and 496 Na2S2O3 5H2O. The solution is made by dissolving one gram of pure copper foil in acid, converting to sulphate, and dissolving in 100 cc water. Hence 10 cc contains 0.1 gm. Cu, equivalent to 0.0836 BrCN; then 1 cc hypo solution corresponds to 0.02 gm. BrCN, and 4.18 hypo solution to 0.0836 gm. BrCN.”
“In testing BrCN solutions, 5 cc are usually taken, Na2CO3 solution is added till alkaline, and then acetic acid till acid. A few crystals of KI, and some starch solution, are then added, and the whole titrated with the standard Na2S2O3 solution.”
“EXAMPLE: If 5 cc BrCN solution took 3.2 cc hypo, then 3.2 x 0.0836/4.18 x 20 = 1.28%
“This method of testing BrCN solutions is different to that used at other mines, where it is customary to titrate direct with Na2S2O3 without first neutralizing the H2SO4.
The following is a series of tests made on a number of 30-lb. BrCN solutions by direct method, A, and, after neutralizing, B.
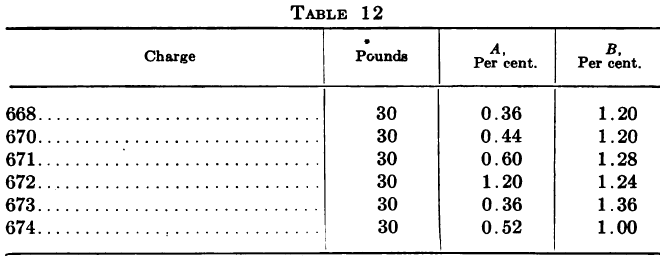
“These show that by direct titration the test is usually low, and also that it gives irregular results, depending on the amount of free H2SO4 present.”
“There seems to have been some doubt as to whether a BrCN solution increases in strength after, say, one hour’s agitation, but tests which were made show that it does increase up to about 8 hours, from 0.56 to 1 % BrCN.”
“It was generally known that if the solution became too alkaline, either through a change in the ores, or the addition of too much lime either to the ore before crushing or after the bromo-cyanide treatment, the extraction by BrCN fell considerably, and for this reason an occasional test of the plant-solution was made for alkalinity, but not until several high tailing-discharges had been observed.”
“Under the old system the KCN did not have sufficient time by itself; the gold in the ore and the KCN residue of each vat were not known, and the amount of BrCN that should be added was more or less a guess. When it is remembered that every 5 lb. of BrCN added to a 50-ton vat represents a cost of 8¢ per ton of ore, that the action of the BrCN can be made just as effective after long KCN treatment, and that excess of BrCN gives no advantage, it will be seen how important it is that the value of the KCN tailing should be known after sufficient agitation (say 12 hr.) and the condition of the vat tested as to alkalinity before adding the BrCN. In any case, the action of the BrCN is of short duration, not exceeding 4 hours, so that if 20 hr. total agitation can be allowed, it is better to give 16 hours with KCN, and then add the BrCN. It is preferable, however, to keep the vat under KCN treatment until the KCN residue is known, then correct the alkalinity, and add the BrCN. This could easily be done with extra vat-capacity.”
“The results of these experiments have suggested certain improvements in bromo-cyaniding, some of which have been adopted, as follows:
- The daily ore sample should be taken in the morning, and assayed as soon as possible, so as to know the value of the ore passing to the vats in the previous 24 hours.
- The pulp should have a long KCN treatment.
- A vat should be kept under KCN treatment till the value of the KCN residue is known.
- The alkalinity of the vat should then be determined and corrected to 0.01 % by H2SO4 before adding BrCN.
- The quantity of BrCN added should then be determined from the value of the KCN residue, the tonnage of the vat, and so forth.
- The lime added to the ore during crushing should be varied according to the alkalinity-test after KCN treatment, so that the plant- solution tests about 0.02%.
- Lime water should be made and added to the vats or to the solution from the presses, instead of adding lime to the vats.
- Metallic iron should be kept out of the pulp as far as possible, as it is both a cyanicide and bromo-cyanicide.”
