The amalgamation assay consists in subjecting a weighed sample of ore to the action of mercury, and separating the latter after the gold is presumed to have become amalgamated. Preferably the original ore or “heads,” the “tails” after amalgamation, and the mercury which has been separated, are all three assayed, as the results then check one another. It is common, however, to determine only two of these factors, usually the original ore and either the amalgamated gold or the tailing. For some reasons, especially in the case of an ore carrying coarse gold, it is preferable to assay the tailing and determine the amount of gold amalgamated, assuming the original value as the sum of these.
Inasmuch as some free gold is quite invisible, and even coarse gold may be very difficult to detect in the presence of a large proportion of sulphurets or heavy pyritic minerals, the amalgamation method has obvious advantages, especially as the results of panning depend so largely on the operator’s judgment and skill in manipulation.
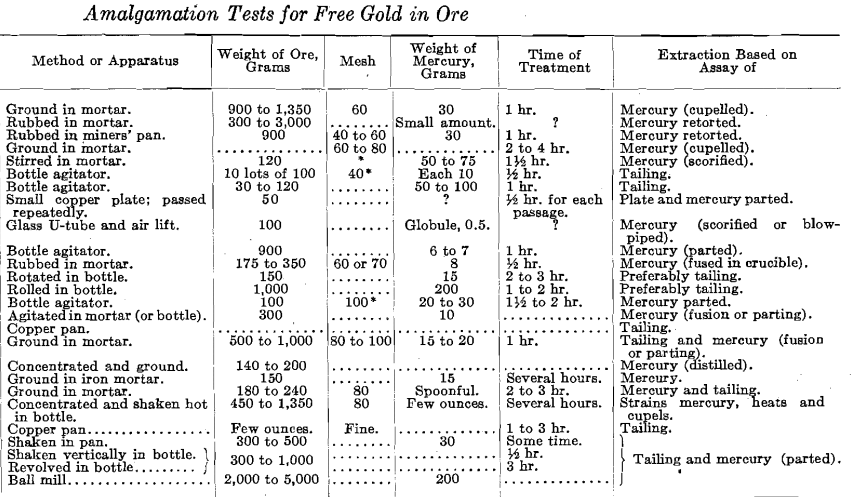
Numerous methods have been published for carrying out the amalgamation assay, but many of the instructions lack precision, leaving so much to the worker’s judgment that they are of little use to the inexperienced, and there is a bewildering variety in the quantities of material and times of treatment prescribed. Such directions as “stir for a few hours, or until completely amalgamated,” are not infrequent.
Some of the authors recommend agitation, others simple rotation in a bottle; some add water to make a thin pulp, while others specify that it must be thick enough to keep the mercury from settling. Some grind the ore with mercury in an iron mortar till it is reduced to an impalpable powder, others merely rub or stir with a wooden pestle, others again insist on a Wedgwood mortar; one uses as much mercury as ore, or even more, while another recommends less than 1%. A few prescribe grinding with a large proportion of sodium amalgam, endeavoring to secure a maximum amalgamation; most, however, aim to obtain results which shall compare fairly with those realizable in a well-equipped gold mill; some try to imitate pan amalgamation by adding various chemicals while grinding in an iron mortar. A few recommend preliminary concentration, amalgamating the concentrate, or at least begin by washing off the slime and lighter minerals; others, also using amounts too large for a single operation, divide them, and use the same mercury on several successive charges of a given ore.
Two pieces of special apparatus may be mentioned. Amalgamated copper pans, or copper-bottomed pans with iron rims, are often recommended for use when the tailing and not the amalgam is to be assayed. Their efficiency is increased by silver-plating, and sometimes by adding a rib or riffle on one side to assist in retaining the mercury. They may be made to fairly imitate the conditions of plate amalgamation. Mechanical bateas or pans suspended by springs are sometimes used for the same purpose. The iron arrastre or Buck’s mortar is cast with an annular trough in which a muller is revolved by hand; in the best forms the muller is divided into several segments; when used with a fairly large proportion of quicksilver it represents closely the action of an amalgamating pan.
In the main there are two principal schools: One relies on the assay of the tailing and pays comparatively little attention to the quality or quantity of mercury used, but removes it carefully by panning; the other depends on the precise determination of the gold recovered as amalgam, and hence demands the use of mercury which is either free from gold, or contains only a small and known amount.
In determining the gold present in the mercury after its contact with the ore, very great differences also occur in the published directions; these are discussed to some extent under the head of determination of gold and silver in amalgam and mercury.
In the accompanying table an attempt has been made to compare the more important details of procedure recommended by various writers. Some of the typical methods are further summarized. Some of the variations noted undoubtedly arise from differences in the objects aimed at; some of the writers have evidently had in mind a process which could be carried out in the field by any prospector, and most of them appear to contemplate only a few occasional determinations, as the methods usually demand too much personal attention to be of every-day application.
Among all the published descriptions found, apparently only one— that of Guess—refers to the amalgamation assay as a real means of plant control; in this case it was used as a regular working guide to mill practice and as the basis for settlements in treating custom ore. Another paper— that of Warwick—deserves special mention, as, in addition to describing amalgamation tests, it treats with considerable detail the examination of an ore to decide the various forms in which the gold may occur.
List of Test Methods:
- A very small piece of amalgamated copper foil is repeatedly subjected to the action of a stream of ore particles passed over it under the surface of water in a flask, the whole sample being passed over it a definite number of times, each passage taking half an hour. The copper plate is then treated with nitric acid and the gold recovered.
- A glass apparatus with an air-lift device causes the ore, mixed with water to a thin pulp, to circulate over a globule of mercury in a narrow U-tube. The mercury is finally volatilized; the residue is fused before the blowpipe, or may be scorified, cupelled, and parted.
- You can also divide a large sample into several lots which are rubbed successively in a mortar, the mercury separated from the first being used again for the second, and so on. Finally it is cleaned and put into a small iron retort and heated to expel the mercury. The gold is recovered by fusing a little test lead in the retort; the lead is then cupelled, “preferably with the blowpipe.”
- Another method uses about 2 lb. (900 g.) of ore crushed to 40 or 60 mesh, and rubs this with an ounce (30 g.) of mercury in a miners’ pan with a wooden pestle. The mercury is “retorted” in a cup-shaped piece of sheet iron, or in a potato, and the gold weighed.
- Weigh out 10 assay tons into a Wedgwood mortar and add water to make a thin pulp. Into this place 10 g. pure redistilled mercury and agitate half an hour. No attrition should be allowed other than that between the particles themselves, and between them and the sides of the mortar. Should the ore contain any ingredient tending to foul the mercury some reagent may be added (sodium hydroxide or hydrochloric acid) to make the mercury more active. If rusty gold is present it is often expedient to add sulphuric acid. The pulp could also be agitated with mercury in a bottle, but this method causes flouring or fine subdivision of the mercury and makes its collection very difficult.
To recover the mercury, pan off the sand and concentrate, taking care not to lose minute globules; even with care a slight loss of mercury ensues. A separating funnel may also be used. The collected mercury is cleaned and weighed, and assayed for gold. The gold thus found is multiplied by the original weight, and divided by the recovered weight, to give the total gold recoverable by amalgamation; the result is calculated per ton of ore.
- Some will also recommend the use of a small separatory funnel with two stopcocks, such as is used in mineralogical work with heavy liquids, for removing mercury from amalgamated tailing.
- One can place in a porcelain mortar 4 assay tons (120 g.) of ore crushed to a suitable size (or treats several such samples at different meshes), adds water enough to make a very thin paste, then 50 to 75 g. of mercury, accurately weighed. The mixture is stirred, not rubbed, with a light pestle of hard wood for hr. It is then diluted, the thin paste poured off, and the mercury washed, dried, and weighed. The loss is indicative of the tendency to “flour” as one can consider amalgamation impracticable if it exceeds 1%. The collected mercury is placed in a scorifier, covered with a second scorifier, placed in a muffle to vaporize, and the residue scorified with lead, cupelled, etc.
- Some metallurgists have described a method used practically for the control of a custom mill, the ore being crushed to 40 mesh, or to agree with the mill practice. From three to ten samples of 100 g. each are put in wide-mouthed stoppered bottles, with 10 g. of mercury and water, and agitated 30 min. A vertical agitator is used, having a 2-in. eccentric and run at 350 rev. per minute. He pans and discards the mercury, using a deep aluminum pan 10 in. in diameter. The concentrate is separated, dried, and assayed; the tailing and slime are dried together and assayed. The values of concentrate and total tailing are subtracted from the original assay to give the amount amalgamated. The returns of a number of mill cleanups are quoted to show their close agreement with these tests,
- And others have used a much larger proportion of mercury than the preceding. He shakes 1 to 4 assay tons of ore with 50 to 100 g. of mercury in a bottle for an hour, by means of a “milk shake” or Gay-Lussac silver-assay apparatus. The bottle is then inverted, most of the mercury poured out, and the remaining globules separated by the use of a copper pan or dish. The proportion of free gold is determined by an assay of the original pulp and final tailing.
It is described in Elements of the Art of Assaying the process of amalgamating previously concentrated ores of native silver (or gold) in an iron mortar, using a wooden pestle and a large proportion of mercury, also adding vinegar and alum. The mercury is finally to be squeezed through thin leather and the solid amalgam distilled in a glass retort on a sand bath. He mentions the danger of precious metal passing into the receiver with the mercury when the fire is too strong. He adds the following caution (following Mortimer’s translation): “If, for want of an apparatus for scorification and coppelling, you have a Mind to indicate by this method, the Quantity of Silver contained in the Body washed; in this case the whole Amalgama must be distilled through the Retort; because Part of the Silver and gold gets through the Leather: Nay, there remains nothing at all of the Silver Or Gold within the Leather, if you use too great a Quantity of Mercury, to extract a small Quantity of these Metals: unless the Mercury be saturated with them, by a like previous Process; and even then, you may be easily deceived as to the Quantity and Quality of the Metal.” . . . “Amalgamation itself is more proper to Gold than to Silver … A true metallic State is required for an Amalgamation, because no Extraction can otherwise be made by Mercury.”
BEFORE ALL THIS…
For the estimation of the value of the visible gold by panning or washing, a weighed sample may be taken, ranging from an ounce to several pounds; a kilogram or 2 lb. is a favorite quantity for laboratory tests, while a prospector often uses a full miners’ pan, say nearly 20 lb. or 10 kg. In the case of rich quartz, ground to about 30 or 40 mesh, a Mexican horn is convenient, using 1 to 8 oz. of material, and a 6-in. frying pan is favored by many prospectors in dry districts. Bateas, plaques, and dishes of various patterns are used for the same purpose. With these, fairly close results may be obtained by using for ocular comparison standards consisting of weighed amounts of gold of similar character as regards purity, fineness of division, and the general shape of the particles, which standards are preferably obtained from gold of the same locality, or the same deposit, as the ore or gravel being tested. Men working in one particular district sometimes become surprisingly expert in thus judging the value of an ore by the eye, the weights of both ore and gold being guessed. On the other hand, many have been led to overestimate the value of ore and gravel from unfamiliar localities, where the gold has been very finely divided or of unusually high silver content, and a novice may be easily misled by the presence of such minerals as lead chromate or molybdate. The presence of “sulphurets” is also often a source of difficulty.
When the visible gold obtained by panning is collected and weighed the results are generally low; when the collected pannings are fused and assayed the results may be high as regards “free” gold, owing to the inclusion of a certain amount of concentrate. Sometimes, after concentration by panning, the concentrate is treated with a little mercury or sodium amalgam, which leads to a combination process.
- Stamp Milling of Gold Ores,
- Trans., m, 645 (1895).
- Trans., xxvi, 187 (1896),
- Metallurgy of Gold.
- Pacific Coast Miner, vol. vii, No. 2, p. 30 (Jan. 10, 1903).
- Mining and Scientific Press, vol. xcv, No. 10, p. 300 (Sept. 7, 1907). Mining World (Chicago), vol. xxxii, No. 6, p. 319 (Feb. 5, 1910). Testing for Metallurgical Processes.
- Cyanid.e Handbook (1910).
- Fulton’s Manual of Fire Assaying, 2d ed. (1911).
- Rand Metallurgical Practice, vol. 1(1912).
- Sampling and Assay of the Precious Metals (1913).
- Elements of the Art of Assaying Metals (Mortimer’s translation, 1764). Fire Assaying (1907).
- Notes on Assaying.
- Manual of Practical Assaying (1893).
- Notes on Assaying (1904).