The use of chlorine gas for the purification of molten gold was first proposed by L. Thompson, and the results of his investigations were published in the Journal of the Society of Arts two years later. He stated that “ it has long been known to chemists, that not only has gold no affinity to chlorine at red heat, but it actually parts with it at that temperature, although previously combined.
This, however, is not the case with those metals with which it is usually alloyed. It offers, therefore, at once an easy and certain means of separation.”
F. B. Miller, the Assayer of the Sydney Mint, applied this property of chlorine to the separation of gold from silver on the large scale, and his process has been in use at Sydney ever since, being particularly suitable for the purpose under the local conditions. Among these conditions may be mentioned the facts that acid is very costly, and that there is a scarcity of silver bullion containing small quantities of gold, while the gold produced in Australia contains but little silver. The result is that the ordinary parting processes would prove very expensive, but the chlorine process can be applied cheaply, as it requires very little acid, and is efficacious in removing small quantities of silver from gold bullion which has not been made up into alloys of definite composition. Before the introduction of the chlorine process no attempt was made to extract the silver from any of the native gold of Australia and New Zealand which was coined at the Sydney Mint. Sovereigns were manufactured containing several per cent, of silver, which replaced part of the copper used as the alloying metal. These sovereigns, some of which are still in existence, can be easily recognised by their pale tint, due to the presence of silver. Such sovereigns have not been manufactured. Besides separating the silver, the chlorine process removes the small quantities of lead, antimony, &c., which render most of the Australian retorted gold brittle, and so in one operation prepares the gold for coinage.
The furnace used is an ordinary melting furnace, such as has been already described. The tile cover of the furnace has a hole in the centre to allow the chlorine tubes to pass through. Clay crucibles are used, the 4-pint size being generally employed, holding about 600 or 700 ozs. of gold; they are placed inside graphite pots to prevent loss by cracking. They are glazed inside by melting borax in them to prevent them from absorbing molten chloride of silver. Graphite crucibles are said to be unsuitable, as silver chloride appears to be reduced, presumably by the hydrogen contained in them, as fast as it is formed. The crucibles are covered by loosely fitting lids, through which the clay pipe-stems of about 3/16 inch bore are passed to the bottom of the crucible for the conveyance of chlorine. The pipe-stem is made red-hot before being introduced into the molten metal, as otherwise it would crack and break off. The chlorine generator consists of a stoneware jar furnished with three necks, and capable of holding from 10 to 15 gallons of liquid. The three openings are fitted with well-secured rubber plugs, through two of which two tubes are passed—viz., the safety tube, which is 8 or 10 feet high, with its open end bent over so as to deliver into a large jar, and the eduction tube, which is closed by a stopcock till it is required. The generator is partly filled with from 70 to 100 lbs. of manganese dioxide in small lumps, an amount which will suffice for many operations; hydrochloric acid is introduced through the safety tube when the gas is required. The generator is warmed by a steam jacket.
The chlorine gas is conveyed in leaden pipes to the furnaces. All joints and connections consist of well-wired india-rubber tubes, which must be protected from direct radiation from the furnace. Screw compression clamps on these rubber tubes enable the supply of chlorine to be regulated to a nicety. When the clamps are closed the gas accumulates and forces the acid up the safety tube into the vessel placed overhead, and so the further generation of gas is prevented. Two such generators and three melting furnaces are enough to refine 2,000 ozs. of gold, containing 10 per cent, of silver.
The generators being in readiness, the crucibles are slowly heated to redness, and the full charge of 600 or 700 ozs. of bullion introduced and melted, 2 or 3 ozs. of borax being sprinkled on its surface or poured on in a molten state. The chlorine is now allowed to pass slowly through the clay pipe to prevent metal from entering it, and the pipe is plunged to the bottom of the molten metal and kept there by means of a weight attached to it. The full stream of chlorine is now turned on and is heard to be bubbling into the molten metal, by which it is completely absorbed, so that no splashing and projection of the metal occurs. A height of 16 to 18 inches in the safety tube corresponds to and balances a height of 1 inch of gold in the refining crucible. The safety tube acts as an index of the pressure in the generator and of the rate of production of the gas: any leakage or the exhaustion of the acid is at once indicated by a fall of the liquid in the tube. Fresh acid is added at intervals as it is required.
When the chlorine is introduced, dense fumes at once arise from the surface of the metal owing to the formation of volatile chlorides of the base metals, which are the first to be attacked : lead gives especially dense fumes, which can be condensed on a cold object held in them. After a time these fumes cease and silver chloride is formed, very little chlorine escaping from the crucible, even if an extremely rapid current is passed into it ; consequently the operation is expedited by every increase in the volume of the current. Towards the end of the operation splashing is more noticeable, and dark brownish-yellow fumes appear, consisting chiefly of free chlorine. The completion of the refining, however, is indicated by a peculiar reddish or brownish-yellow stain which is imparted to a piece of white tobacco-pipe when exposed to the action of the fumes for a moment. It is suggested by Prof. S. B. Christy that the stain contains gold. This stain appears in about one hour and a-half from the start, when 600 ozs. of gold, containing 10 per cent, of silver, are being subjected to treatment. The current of gas is then at once stopped, and the crucible lifted out of the furnace and allowed to cool sufficiently for the gold to solidify. Probably, if the operation were continued after the appearance of the brown stain, losses of gold by volatilisation would occur.
The chloride of silver, still molten, and floating on the top of the gold, is then poured off into iron moulds, and the crucible inverted on an iron table, when the red-hot cone of gold falls out. This is now fine, and after any adherent chloride of silver has been detached from it by scraping, it simply requires melting into ingots, 98 per cent, of the gold being thus at once rendered available for use. The remainder of the gold is contained in the chloride of silver, partly in the form of entangled shots of metal, but chiefly as a double chloride of silver and gold. It was formerly recovered by melting the chloride with about 10 per cent, of metallic silver, rolled to about 1/8 inch in thickness. The gold is reduced by the silver and alloys with the excess settling to the bottom of the pot where it solidifies after ten minutes cooling, so that the chloride of silver can be poured off into large iron moulds, slabs suitable for reduction being thus formed.
It was found at the Sydney Mint, that the above method of separating the gold from the silver chloride was subject to several disadvantages. In particular, although on a small scale the amount of gold in the silver could be reduced to from 0.3 to 1.0 per 1,000 by careful and continuous stirring with silver foil for a great length of time, nevertheless in practice on a large scale the results varied greatly, and the silver bullion produced usually contained from 10 to 25 parts of gold per 1,000. Several reducing agents, such as argol, resin, hydrogen, and coal gas were successively tried but were not found to give good results. Finally, the application of soda carbonate, which had been proposed by Leibius in 1868, was adopted, the method of procedure being originally as follows :— “ The argentic chloride is covered by a layer of fused borax, about ¼ inch thick, and when all is well fused, the powdered soda is sprinkled on the top of the borax, without stirring, as rapidly as the ensuing action will admit. Occasionally the top layer is dipped with a stirrer slightly underneath the molten argentic chloride, without stirring the latter. When all the necessary soda is added and the action is nearly over, the pot is covered with a lid, and left for about ten to twenty minutes to increased heat, and, when the contents are quite liquid, the pot is lifted out of the fire without previous stirring, and allowed to cool, so as to enable the argentic chloride to be poured off from the gold button at the bottom of the pot.
“ Although in several experiments all but 0.1 of gold per 1,000 was eliminated from the silver bullion produced, in no case is every trace of gold removed in one operation. To free the argentic chloride entirely from gold, producing therefore silver bullion free from gold, was, however, accomplished by subjecting the argentic chloride to a second treatment, with a small quantity of soda, in a separate boraxed clay pot, similar to the first operation.
“ A convenient quantity of argentic chloride, to be treated in a No. 18 French clay pot, was found to be 230 ozs. The amount of soda required for 230 ozs. of chloride may range from 16 to 20 ozs. Less than 16 ozs. leaves too much gold in the silver, while more than 20 ozs. produces a very silvery gold button, and yet without completely freeing the argentic chloride from gold.
“ The use of 18 ozs. of soda for 230 ozs. of chloride produces a gold button weighing between 30 and 35 ozs., assaying about 920 to 930, and leaves from 0.5 to 1.0 part of gold in 1,000 parts of silver bullion produced.
“ With 20 ozs. of soda the results were:—Gold, about 35 ozs., assay 870-880; gold left in the silver bullion produced from 0.2 to 0.5 per 1,000.
“ With 16 ozs. of soda:—Gold from 30 to 33 ozs., assay 940-950; gold left in the silver bullion, from 1.0 to 2.0 per 1,000, and sometimes as much as 6.0 per 1,000.
“ To free the argentic chloride from gold, a second treatment with 3 ozs. of soda per pot of 200 ozs. chloride, containing but a minute quantity of gold, will always be found to answer, the only care required being gradual application of the soda and enough heat at the end of the operation.”
The time required for the two operations is about half an hour.
“ The presence of a large proportion of chloride of copper has been found to prolong the operation considerably on account of oxide of copper being formed on addition of soda, as a much greater heat is required in order to fuse the whole mass. The argentic chloride produced from base gold alloys would contain a large proportion of chloride of copper, &c., and it would be better, therefore, to reduce it direct, and dissolve the reduced metals in acid, to separate gold and silver therefrom.” The silver chloride may be assayed for gold by cupellation with lead foil and subsequent parting.
The method just described was adopted at the Sydney Mint, and at the Melbourne Mint in the following year, with excellent results.
The process of reduction of the silver chloride was devised by A. Leibius, of the Sydney Mint. In this process, 1,400 ozs. of silver chloride are reduced in 24 hours by the apparatus, of which the following is a brief description:
Seven zinc plates, each 14 inches long, 12 inches wide, and ½ inch thick, are supported about 1¼ inches apart in a vertical position in slots in a wooden frame. Six slabs of argentic chloride, each 12 inches long, 10 inches wide, and ¾ inch thick, are suspended by loops made of silver bands, in such a way that each slab is placed between two of the zinc plates and separated from them by spaces of about ¼ inch. The silver loops are connected with silver bands on which the zinc plates rest, so that there is metallic connection between the slabs of chloride and the zinc plates. The whole is now plunged into water, to which some of the liquor from a previous operation containing chloride of zinc in solution is added as an exciting agent. Galvanic action soon begins, the liquor gets gradually warmer and a strong current is discernible. The silver chloride is gradually reduced to metallic silver, the slabs undergoing no alteration of form, and the zinc is dissolved. The slabs of silver chloride are generally free from most of the base metals, but copper, if present in the original alloy, is not volatilised in the crucible, and its chloride remains mixed with that of the silver. The two metals are now reduced together. When all action has ceased, the slabs of cupreous silver are lifted out and boiled, first in acidulated water and then in pure water, while still suspended in their silver loops. The porous metal is now ready for melting. As no acid is used the amount of zinc consumed is the theoretical quantity required by the equations:
2AgCl + Zn = ZnCl2 + 2Ag
CuCl2 + Zn = ZnCl2 + Cu
The weight of zinc consumed usually amounts to from 24 to 25 per cent, of the weight of the slabs of fused chloride. The zinc plates are used over again until worn too thin for safety, after which they are melted-up and cast into new plates. They suffer no loss if the apparatus is left untouched for any length of time after the whole of the silver has been reduced.
At the Melbourne Mint in the year 1889, the zinc plates employed as described above were replaced by sheets of iron with satisfactory results. “ Upon the reducing bath being heated with steam, the chloride of copper dissolving, disengages itself freely from the slabs of chloride of silver, and coming into contact with the iron is reduced, and the metallic copper falls to the bottom of the bath in large quantities, leaving the reduced silver in a much cleaner state than when zinc was used. The noxious fumes which were formerly given off on the melting of the reduced silver sponge are also avoided.”
The Chlorine Process
The following description has been kindly supplied by Mr. Francis R. Power, the Assayer at the Royal Mint, Melbourne, by permission of the Deputy Master. It gives the exact methods and apparatus in use in the early part of 1896, and has been amended by Mr. Power so as to include all changes introduced up to March, 1905. As will be seen, the methods differ considerably from those described above, and from the practice at Sydney.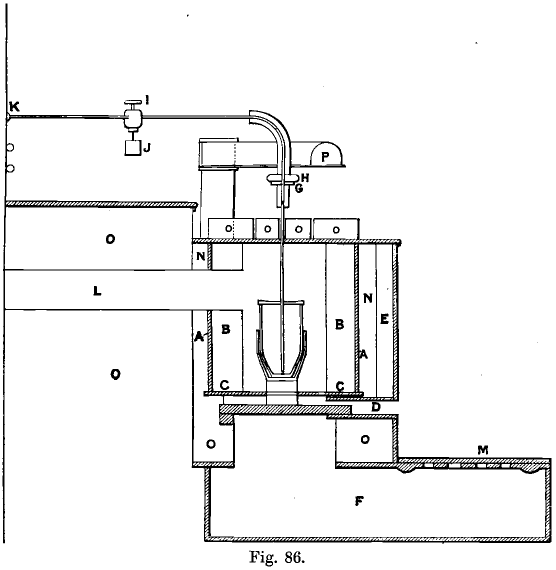
Gold Refining Furnace
These are thirteen in number. They are built cylindrically (see Fig. 86, in which one of these furnaces is shown in section, with crucible and pipe-stem in position), being more compact in this form, more easily cleaned from clinker, and more economical in fuel than the square ones. They are 12 inches in diameter and 21 inches deep. The five firebars, 1½ inches square and 18 inches long, are set in a cast-iron box, D, 12 inches by 2 inches, which passes through the brickwork in front of the furnace, the other ends of the firebars resting on an iron bar set in the brickwork at the back of the furnace. The bars are 6 inches above the floor. The draught is obtained through a grating in the floor, which covers a portion of the ashpit, over which there slides a cast-iron plate, M, ¼ inch thick, for regulating the admission of air, and pivotted in one corner. The flue, L, is 6 inches square, and communicates with a series of five condensing chambers, 8 feet by 8 feet by 5 feet, running the length of the furnaces (42 feet), all communicating and leading to the stack, 80 feet high, common to refining and melting furnaces, which are twenty-one in all. There are three furnace covers, two of them 20 inches by 6½ inches, the third a little smaller, and all are bound with iron. The middle one is perforated by a 1-inch hole, through which the chlorine delivery pipe passes. Glenboig arched firebricks, B, 9 inches by 4½- inches, and tapering from 2¾ inches to 2 inches, are used for lining the furnaces, and are set with touching joints in an iron cylinder, A, 21½ inches in diameter, and at least ¼ to 3/8 of an inch thick, which is supported by a cast-iron plate, C, 5/8 of an inch thick, and 22 inches in diameter, with a 12-inch hole in the centre. This plate is supported by the brickwork which forms the foundation. The ashpit is a cast-iron flanged box, easily cleaned in case of an accident. Round the iron cylinder concrete, N, is rammed, the front iron plate of the furnace being shifted 2 or 3 inches in, until this is set and then moved out, thus providing an air space, E, and keeping the plates cooler. The furnace top is a plate of cast iron and, so as to facilitate repairs, should be in two pieces for each furnace, halved into one another, the hole being slightly bossed at the edge so that the firetiles may run easily on them. One piece has a hole 6 inches in diameter over which the swing ventilating hood, P, is placed by which the pot is covered when removed from the fire. This hood communicates by a passage through the brick¬work with the flue. The cylindrical furnace is calculated to last for three years, the square ones lasting only eighteen months and taking three hours to reline, while the cylindrical ones take one hour.
Preliminary Refining
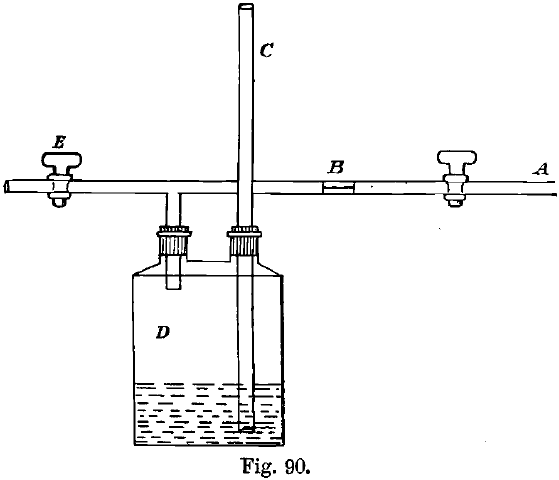
Though the main object of the chlorine process as used at Melbourne is to produce nearly fine gold, which on alloying with copper will be tough, it is frequently employed to enable gold containing much base metal to be partially refined [so as to give a correct assay, or to enable a check assay to be made on finer metal, where the appearance of the original clips and ingot make it doubtful if a trustworthy assay is possible]. In the case of ingots containing much silver, the operation has to be carefully conducted, otherwise some silver may become converted to chloride. In partial refining it is necessary to vary the pressure of the gas according to the weight of the ingot. The pressure may be reduced automatically from the full pressure as delivered from the generator as follows (see Fig. 90):
A is the full pressure main.
B is an ebonite plug, with a pin-hole drilled through the centre, inserted in A.
C is a glass pressure gauge reaching to the bottom of a Woulff’s bottle, D, which contains water, and is of about a litre capacity.
The gas passes from A through B and into D. The tap, E, allows it to pass into the melted metal, through which it bubbles as soon as the pressure in D is sufficiently great to overcome its resistance. Test pieces are dipped from time to time to determine the extent to which the refining has gone.
In a recent trial with gold-silver alloy, the silver was removed at the rate of 1 oz. per minute.
The Miller Process at the Sydney Mint
Mr. J. M’Cutcheon, late Assayer at the Sydney Mint, wrote in 1897 that the process of freeing the chlorides from gold in use was as follows :—“ The chlorides produced during the operation are separated into two classes, termed ‘balers’ and ‘non-balers.’ The first is that portion baled, or rather ladled, out during the operation, to prevent overflow; this is re-melted in quantities of 350 ozs., and whilst in the molten state, half a pound of bicarbonate of soda is projected on the surface. This has the effect of reducing some of the chloride, and the metal in sinking to the bottom of the pot carries with it all the gold. The ‘ non-balers,’ or that portion of chloride which is poured off the refined gold when it has set, is treated as above, but 7 ozs. of granulated zinc is used instead of the bicarbonate.
“The chlorides are poured into slabs, and are now ready for the reduction process, in which the silver loops formerly used have been abandoned, iron plates being now used instead of zinc ; the water is acidulated with hydrochloric acid.”
The bullion treated at the Sydney Mint during 1895 contained—gold, 832.1; silver, 135.5; and base metals, 32.4 parts per 1,000.
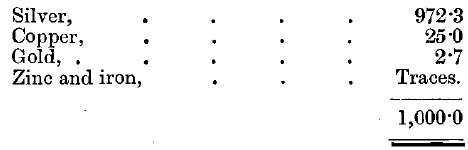
The average fineness of the gold produced by this method at Sydney in the period of nine years from 1884 to 1892 was 995.9. The remainder is silver, which apparently cannot be profitably removed by chlorine or by any other method. The average fineness of the silver produced at Sydney was about 970 in 1893, the fineness of individual bars varying from 917 to 987. The alloying metal in the silver bars is almost all copper. The gold retained by the silver formerly amounted on an average to 1.3 parts per 1,000, but it has since been materially reduced and is now a mere trace. Analysis of the silver resulting from the refinage of gold, known originally to have contained, among the base metals in the alloy, copper, lead, antimony, arsenic, and iron, gave the following results:
The losses of gold in the course of the process are stated to be very small, varying from 0.11 to 0.19 per 1,000; this is considerably less than would have been lost by merely toughening the gold with corrosive sublimate without parting it from the silver. The loss of silver at Sydney was about 4.25 per cent, in 1895. These losses are reduced if the amounts recovered from the flue-dust and from the ground-up crucibles are taken into account. The total cost of the process is about 1d. per oz. of crude gold at Sydney, and was about 0.65d, per oz, in Melbourne in 1873, but has since been reduced. The loss of gold by volatilisation is probably prevented from reaching the large amounts which might be expected from the results of Christy and of the author (see p. 22) by the fact that, during the whole time that the chlorine is being passed, silver and base metals are present, and, by absorbing the gas, protect the gold from its action.
Professor Thomas Price, who treated some of the Californian gold bullion by this method on a working scale in his laboratory in San Francisco, states that, with Californian gold, which generally contains more silver than Australian gold, the gold taken up by the chloride of silver amounted to 5 per cent., and even to 10 per cent, of the total weight of the gold. For this reason, and on account of the large amount of silver bullion in the San Francisco market requiring parting, Professor Price considers that the Miller process, while technically successful with Californian gold, is hardly able to compete commercially with the ordinary sulphuric acid process. He suggests that it might be well adapted for refining the nearly pure brittle gold produced at chlorination works, where chlorine is at hand and other methods of refining are not convenient.
