The how-to method of removing oxidisable metals by means of an oxidising agent has been practised for centuries. The operation has already been described in the cementation process with nitre, and the gaseous method of removing antimony from gold. The ordinary cupellation operations are also on the same lines. When oxidising fluxes are used, their action is limited, since the oxygen they contain is rapidly evolved when brought into contact with gold at a high temperature—moreover, it only acts on a portion of the metal on the surface at the time. When air is allowed to pass, or is blown over molten metals a large surface must be exposed, as in a cupelling furnace, for the operation to be reasonably rapid.
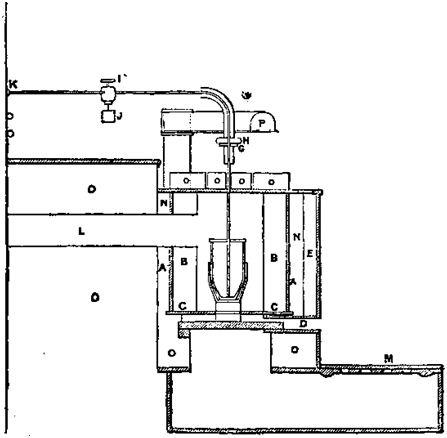
In the year 1894 the author had to deal with considerable quantities of gold sent down from the Gippsland mines; much of the gold was exceedingly impure, and it was found that the action of ordinary refining fluxes, although good up to a certain point, never served to remove anything near the whole of the base metals present. Experiments were made by blowing air on to the surface of the molten metal, to promote oxidation, and removing the oxides by means of borax. This proved to be too slow. Air was then blown into the molten mass, but although it appeared to act rapidly, there was difficulty in keeping the pressure constant. As a result, on two occasions the gold was projected out of the pot, and the trouble of collecting it out of the coke and ashes was not compensated by the results obtained. For small amounts, the method took up too much time, and required too much attention to be persevered with. Had a cylinder of oxygen been available, this would have been tried, instead of air; but so far as experiments went at the time, it appeared that the process, while it would serve for the oxidation of impurities, yet would be attended with so much spitting, or projection of fine particles of gold, as to be practically of no value. This opinion was expressed in discussing this question in 1901. Dr. Kirk Rosef in 1904 ably deals with this subject. Oxygen from cylinders was allowed to escape through composition pipes of about 1/8 inch bore to a clay pipe dipping to the bottom of the pot. The connections were made of rubber tubing, fastened on with rubber solution. The pipes used were those made for the Miller chlorine process. They are made of clay, about 1/8 inch bore. The oxides formed corroded them quickly at the mouth of the pipe at the beginning of the experiment. When air was used it had little effect on the pipes.
The stream of gas was regulated by a screw clip just above the crucible; a small stream was turned on, and the pipe slowly heated in the furnace, and then gradually inserted into the molten metal. The passage of the gas could be felt by the throbbing of the rubber tube. Dr. Rose took the precaution to cover his crucible, so that any projected shots of gold would be saved.
The molten metal was covered with sand and borax, so that the oxides of the base metals might form a fluid slag, without undue attack on the pot. The amount of sand added was calculated to provide a fluid slag on the completion of the experiment.
The pots used were made of plumbago, with a fireclay lining. The graphite in a plumbago pot would undo the work of the oxygen.
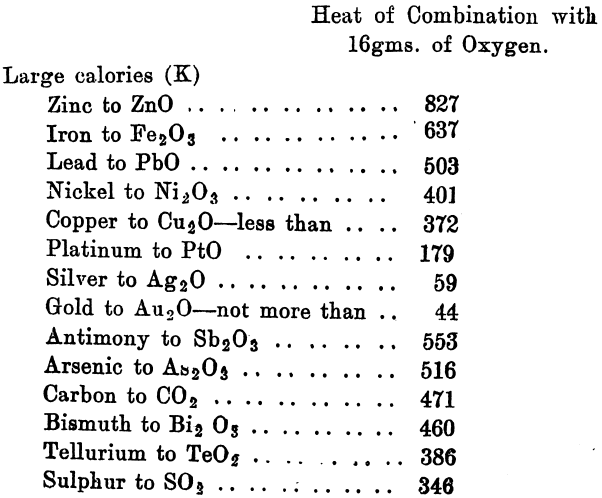
Dr. Rose first studied the effect produced when oxygen is blown through molten metals or alloys, and compounds of these, and refers specially to the refining of such low grade gold bullion as is frequently produced from zinc boxes in the cyanide process. The effect of blowing oxygen through molten gold would result in the retention of part of the oxygen, and if an acid slag were present there would probably be formed a silicate of gold. The amount, however, under the conditions necessary for refining would be insignificant. Silver, on the other hand, absorbs large quantities of oxygen, when melted in the air it absorbs about 20 times its volume, or 0.27 per cent, by weight. This would correspond to 3.64 per cent, of silver oxide. By the law of partial pressures, much more should be dissolved, if it is a matter of mere solution, in oxygen. If at a high temperature, in presence of an acid slag, such as borax or silica, the silver in the form of oxide, unites with these materials, and passes into the slag as a borate or silicate. This was abundantly proved by experiment. Copper, lead, nickel, iron and zinc are readily oxidised when molten by a stream of oxygen, and if a suitable acid flux is provided the oxides of these metals will readily form silicates at the moment of conversion.
Tellurium and selenium pass off at a high temperature as oxides, unless the metal containing them is covered with slag, in which case they are retained to a greater or less extent. Antimony behaves similarly.
Dr. Rose believes that platinum, palladium, rhodium, iridium could be oxidised by this method at a high temperature and slagged off, although it is possible that some of them would be partly protected by silver.
Some important conclusions are arrived at, which tend to alter the current belief on the cause of the removal of base metals from bullion by oxidisation. It is well known that cuprous oxide forms when copper is melted in contact with air. The cuprous oxide remains dissolved in the molten metal. If the mass is allowed to solidify, this oxide separates out and remains scattered through the mass in small particles. If a large quantity forms, over 9 per cent, of Cu2O, then the excess rises to the surface, and can be slagged off. The cuprous oxide is therefore a carrier of oxygen to more oxidisable metals.
Silver in the same way probably absorbs oxygen, and becomes silver oxide Ag2O, and on passing a current of oxygen through molten silver, silver oxide can be slagged off if borax is over the surface. It can easily be seen that if iron, nickel, lead, and other oxidisable metals are present, that oxygen will be supplied to them by these oxygen carriers, and the visible effect will be that they are transformed into oxides.
Cu2O+Fe=2Cu +FeO.
Ag2O+Pb=2Ag+PbO.
While an excess of base metals is present, the silver oxide is reduced as fast as it is formed, but towards the finish of the operation silver oxide is carried away. One notable result is that while most of the base metals are oxidised by silver oxide, and the silver reduced thereby, forming protective agents against slagging off of silver, yet copper does not exert this protective influence; in other words, much silver can be removed by an oxidising process into the slag, while part of the copper still remains in the bullion.
Generally speaking, the metals are oxidised successively, each acting as a protective agent for the other; the order of their removal is not sharply defined, but is in general accordance with their heat formations, zinc being first eliminated.
The slag making material used consisted of borax and sand; the former was found to be too corrosive, but a suitable admixture of the two, mainly depending on the amount or bases present, will give a fluid and non-corrosive slag. The general formula given is 3(Na2O. B2O3+6RO) + (B2O3) 3SiO2, where R = Ca, Mg, Pb, Zn, Cu, Sb, 2/3Fe, 2/3Ni.
The shots of gold can be recovered from the slag by concentration, and the silver and the balance of the gold by melting with iron and carbon.
By using air instead of oxygen the operation was much simpler, and required very little attention. Practically the only cost would be in keeping the metals melted while the operation was proceeding, and if this were done in a gas- heated furnace, holding a number of pots, it would be much cheaper than most of the processes now in operation.
At the time Dr. Rose wrote his paper he was not aware of any work having been done on the subject at all, but it subsequently transpired that this method of refining crude bullion was actually practised at Menzies, in W.A., and a patent for it taken out in 1896 by Messrs. Manton and Rayfield. In January, 1906, I learnt that experimental work was done at the Great Boulder mine on a large scale. A conical cast-iron pot, shaped like a slag pot, was lined with fire bricks and heated by means of a charcoal fire. The bullion was melted in a Faber du Faur furnace, and about 6000oz. poured into the converter. Unfortunately the slag from the melted gold was not first eliminated, and this on entering the converter attacked the ashes and charcoal remaining and boiled over. The clay pipes provided for forcing the air in also gave trouble, and the experiment failed for want of attention to preliminary detail. It was said to be very successful on a small scale, but lead was usually added to help to slag off the copper as oxide.
The method of refining by means of oxygen gas or air has advantages for certain grades of bullion. The methods at present available are cupellation and treatment by chlorine gas. The former involves some expense in erection and maintenance, and bye-products have to be worked up. The latter is expensive in operation, and noxious fumes are evolved.
The natural places for the development of the oxygen refining process are the mints and refining establishments. In spite of the comparative ease with which it can be worked by those accustomed to handling and melting metals, there would be much danger of loss in most small works if this process were generally introduced. These losses would amount to more than the saving of refining charges. The fact that the process was tried in Western Australia does not amount to much, for the bullion for the most part produced there is fairly high grade, and efforts have been directed rather to the preliminary treatment in refining than to the melting down of all the dirty accumulations in the zinc boxes, and the subsequent elimination of base metals.