The ordinary gangue of most ores (silica and silicates of the alkalies and alkaline earths) exercises no direct influence on the cyanide solution. The carbonates of the alkaline earths are also probably without influence. The decomposing effects of sulphides of the heavy metals vary with the physical state of the sulphides.
Metallurgists found that dilute cyanide solutions exercise a “ selective action ” in dissolving gold and silver in whatever form they may be present, in preference to sulphides or other salts of the base metals. There are exceptions to this rule, some of which are noted in the sequel. “ For instance, cyanide of potassium solution has a strong tendency to dissolve precipitated sulphide of zinc, but its action on the natural sulphide of zinc, blende, is almost nil. The same holds for compounds of iron, and thus we prove selective action by the average result of a series of experiments on ores. Let us suppose a pyritic ore containing about 7 per cent, of iron and 8 per cent, of sulphur with about 1 oz. of gold to the ton. After grinding, this ore is treated with a solution containing about 1 per cent, of cyanide of potassium. The most of the gold will be dissolved and the rest of the ore left practically untouched. It is obvious that the amount of cyanogen contained in the solution is insufficient to combine with the iron present in the ore, yet, notwithstanding the much greater mass of iron sulphide present and open to attack, it is the gold that is selected for action by the cyanide solution. Taking the average result of our work we find that a higher percentage of gold than of silver is extracted, which justifies us in concluding that the selective action is greater on the former than on the latter. One of the ores on which our early investigations were done was composed as under:

In this ore we had an extraction of gold 85 per cent., silver 50 per cent., for a consumption of cyanide of about 0.45 per cent., and investigations showed that the action was directed in the order, gold, silver, iron, zinc, copper. For the amount of cyanide consumed it is obvious that the amount of base metals dissolved must have been very slight.
“The consumption of cyanide on fresh concentrates varies naturally with the composition of the concentrates. In many cases it is less than 0.2 per cent, of their weight. When the concentrates contain marcasite there is a greater consumption of cyanide than when the pyrites is entirely of the ordinary yellow cubical description. The presence of compounds of copper, physically soft, also tends to increase the consumption.”
Increase of consumption with increase in strength of cyanide is shown in the following table, given by MacArthur. When one of the stronger solutions is used, the amount of cyanide consumed is equal to the whole amount present in the weaker solutions:—

It has been laid down as a general rule that oxides, hydrates, carbonates, sulphates and sulphides of those metals which are electro-positive to gold in cyanide solutions are dissolved more rapidly than the last named metal, whether it is present in the metallic form or contained in its commonly occurring salts.
This rule certainly applies to the precipitated salts commonly occurring in the laboratory, but J. S. MacArthur has shown that the case may be quite different when the naturally occurring minerals are concerned. Thus, not only is precipitated sulphide of copper rapidly dissolved, but also a sooty form of the same substance occasionally met with as a mineral occurring in ores. On the other hand, fused copper matte is scarcely acted on at all, and in a great many cases the same may be said of the hard dense sulphides of copper usually found in nature. Sulphide of zinc exhibits the same differences of behaviour: the “ black-jack ” concentrates of the Ravenswood Mine, Queensland, can be treated with good results, little zinc being dissolved. Again, oxide of copper, if freshly precipitated, is strongly acted on by the cyanide, but if it is heated to dull redness in a muffle it becomes insoluble, and a large excess of this material added to a gold ore makes no difference in the percentage of extraction, while the consumption of cyanide is not increased by its presence.
The action of cyanide solutions on sulphide of silver is similarly dependent almost entirely on its physical state. Experiments conducted by Fresenius showed that if a weak solution of silver nitrate is precipitated by a weak solution of sulphide of ammonium, the resulting sulphide of silver is soluble in a weak solution of cyanide of potassium. On the other hand, if strong solutions of silver nitrate and ammonium sulphide are mixed together, the precipitated silver sulphide can only be dissolved by concentrated solutions of potassic cyanide, and if this solution is subsequently diluted with water the sulphide of silver is precipitated. These results tend to show that sulphide of silver is not decomposed by cyanide of potassium, but is held in solution by it as a hydrate. Similar peculiarities in the behaviour of sulphide of silver are observed in practice when ores are being treated, and in this case an increase in the strength of the solution quickens the action of the potassium cyanide even though dilute solutions may be eventually efficacious if enough time is allowed. A number of experiments made on various salts of silver point to the following conclusions:
Silver chloride is readily soluble in cyanide, and the arseniate is also rapidly dissolved. Silver sulphide and antimonide are less easily acted on, but are not so refractory as metallic (cement) silver. The presence of copper salts appears to exercise a detrimental action on the solubility of silver sulphide.
Prof. Christy’s experiments on the electromotive force of metals and minerals in cyanide solutions, compared with:
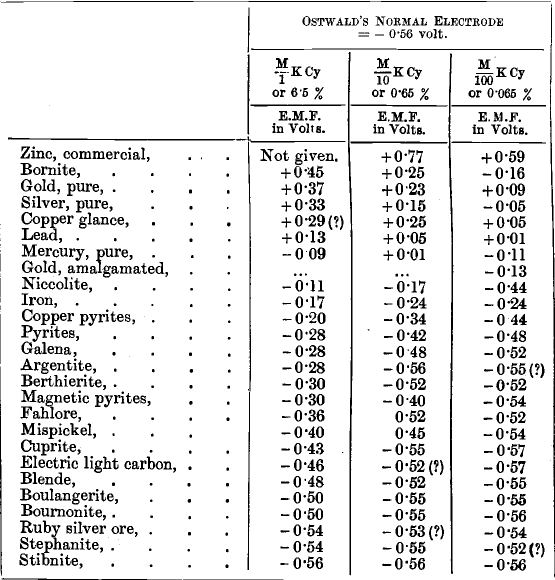
Ostwald’s normal electrode, show that many minerals tend to be less rapidly dissolved than pure metallic gold. The foregoing table is abridged from the one given by him.
Prof. Christy observes that in testing the minerals it was difficult to get good electrical contact between the conducting platinum wires and the rough surface of the mineral fragment, so that the results are only provisional, especially as the resistance in some cases, such as zinc blende, stibnite, &c., was very high. This would tend to make the results for the minerals too low. Nevertheless, the table is interesting, and shows, for example, that copper pyrites has hardly more tendency to go into solution than pure pyrites, while bornite and copper glance have a strong tendency to set up electric currents and to dissolve. It is clear from the table that, in the case of the samples which Christy used, pure copper pyrites, galena, argentite, magnetic pyrites, fahlore, mispickel, blende, boulangerite, bournonite, ruby silver ore, stephanite, and stibnite, when free from their oxidation products, are apparently little acted on by cyanide solutions, but he gives no information as to the physical condition of the minerals tested.
