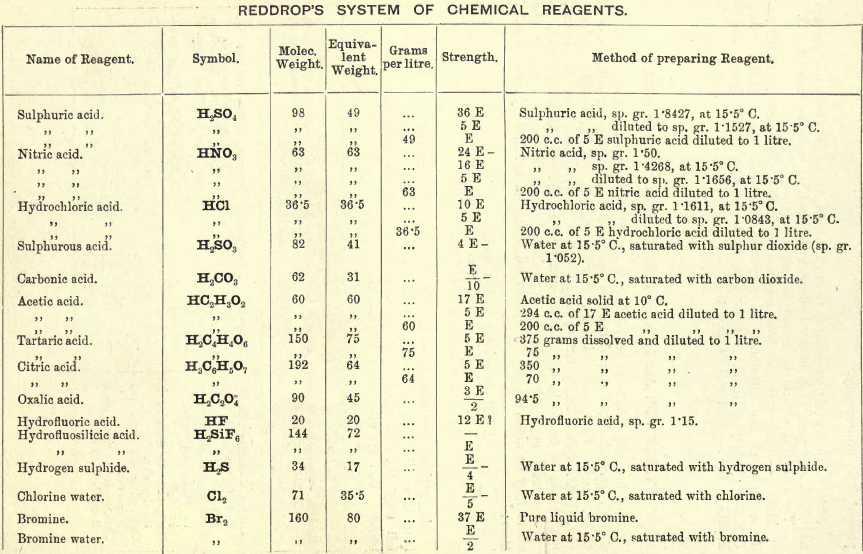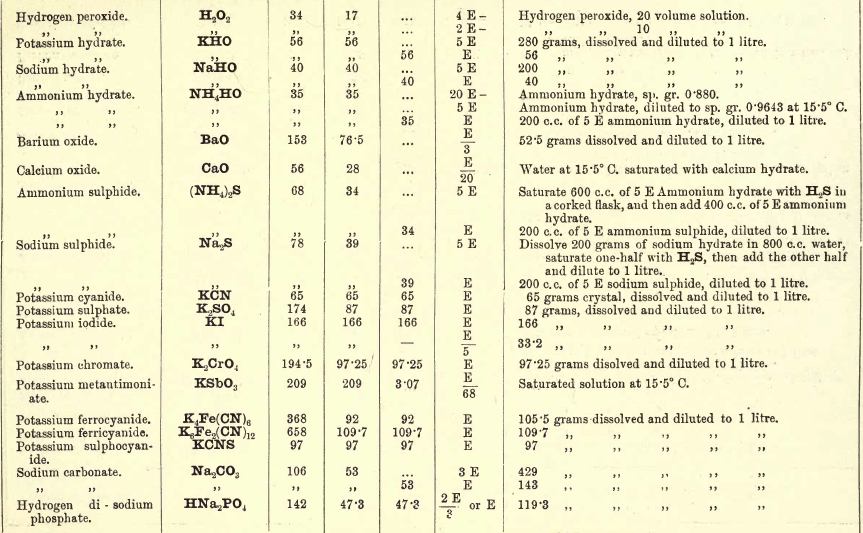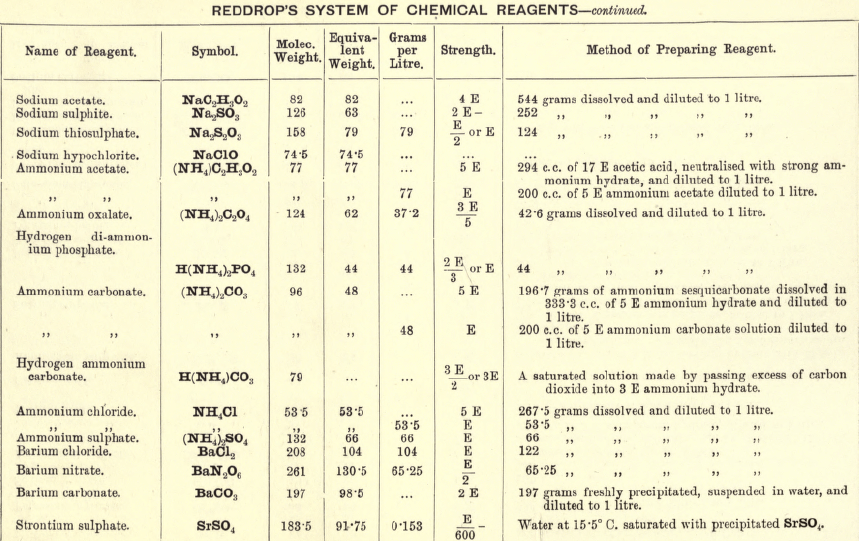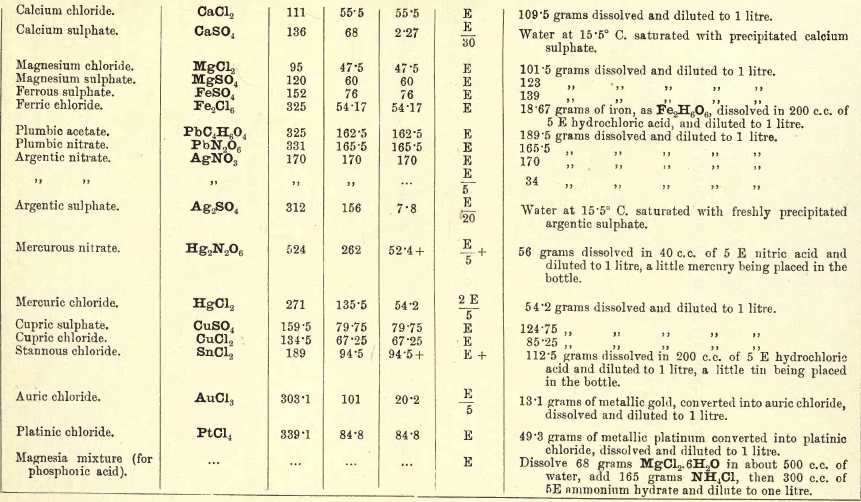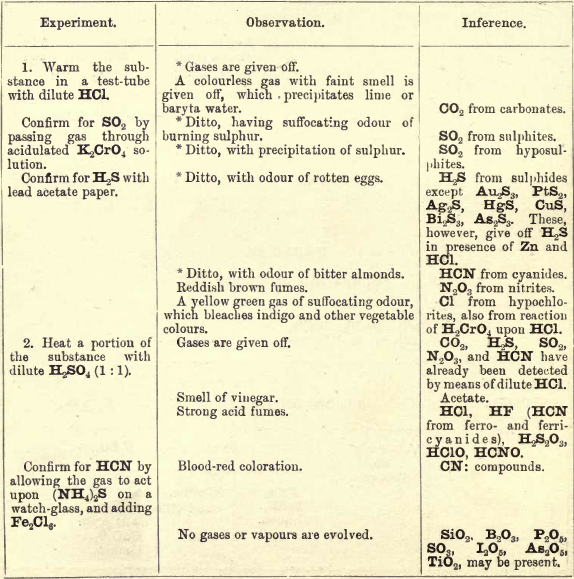
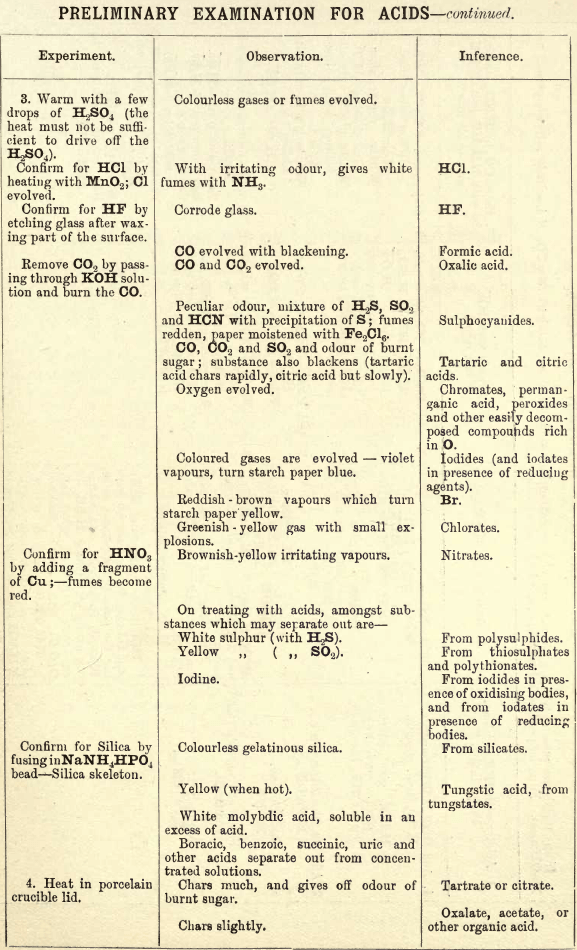
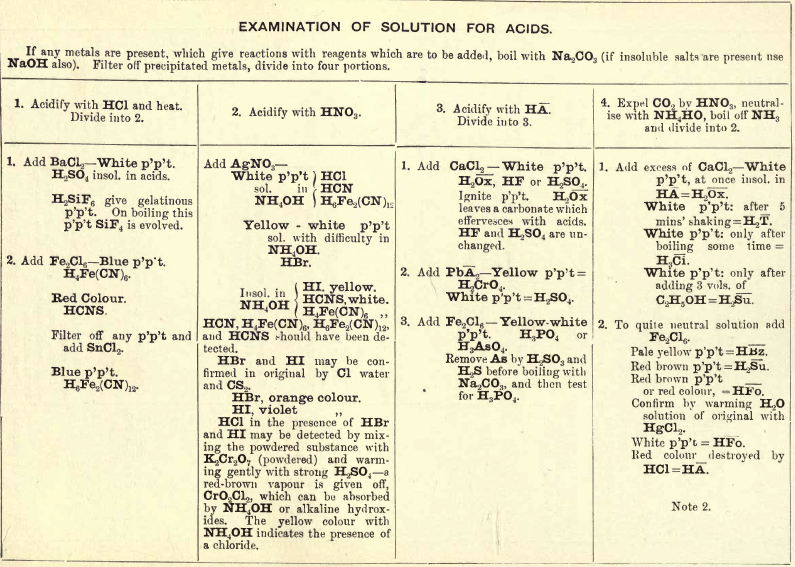
Note 1. Do not boil with Na2CO3 unless necessary. If only alkalies are present it is unnecessary.
Note 2. If organic acids and Groups I. and II. or H2CrO4 are present add HCl, and pass H2S before boiling with Na2CO3 to make solution (4).
The following should be tested for separately:—
HNO3.—Black ring test, with solution of FeSO4 and H2SO4 H3BO3.— To a little of the substance in a watch-glass add strong H2SO4, then C2H5OH; apply a light. If alcohol burns with a green-edged flame = H3BO3. HF.—Sand and H2SO4 test.
TABLE FOR ANALYSIS OF INSOLUBLE SUBSTANCES
The insoluble residue remaining after treating with acids should be thoroughly washed and then dried. It may contain the following substances— C, S, AgCl, SiO2 and Silicates, BaSO4, SrSO4, Al2O3, Cr2O3, Chrome Iron (FeO.Cr2O3), CaF2, SnO2, Sb2O4. The following also may be present, but should be found in solution as well—PbCl2, PbSO4, CaSO4, Fe2O3.
A careful preliminary examination should be made first. Some of the above may be dissolved out by special solvents, and tested for in the filtrate, thus:
PbCl2 is soluble in hot water.
PbSO4 is soluble in ammonium acetate.
AgCl is soluble iu NH4OH or KCN.
After these preliminary tests, mix the dry substance with from three to four times its bulk of fusion mixture (Na2CO3 + K2CO3) and fuse on Pt foil (Ag and Pb must first be removed) until the mass is in tranquil fusion. Boil up with hot-water, filter.