Assaying has for its object the determination of the quantities of those constituents of a material which add to or detract from its value in the arts and manufactures. The methods of assaying are mainly those of analytical chemistry, and are limited by various practical considerations to the determination of the constituent of a small parcel, which is frequently only a few grains, and rarely more than a few ounces, in weight. From these determinations calculations are made, which have reference to a mass of material of, perhaps, hundreds of tons. But in all cases, whether the mass under consideration be large or small, whether the material be obtained by mining, grown, or manufactured, the assayer is supposed to receive a small quantity, called “ the sample,” which is, or ought to be, the exact counterpart of the mass of material that is being dealt with. The taking and making of this sample is termed “ sampling” ; and the men whose special work it is to select such samples are “ the samplers.”
But although “sampling” is thus distinct from “ assaying,” the assayer should be familiar with the principles of sampling, and rigorous in the application of these principles in the selecting, from the sample sent him, that smaller portion upon which he performs his operations.
Sampling
In the case of gases, there is absolutely no trouble in mixing. The only difficulty is in drawing off a fair sample where, as in flues, the body of the gas is in motion, and varies a little in composition from time to time. In this case, care must be taken to draw off uniformly a sufficient volume of the gas during a prolonged period ; any portion of this larger volume may then be taken for the analytical operation.
In the case of liquids, which mix more or less easily—and this class includes metals, &c., in the state of fusion—more or less severe agitation, followed by the immediate withdrawal of a portion, will yield a fairly representative sample.
In the case of solids, the whole mass must be crushed, and, if not already of fairly uniform quality, mixed, before sampling can take place. Most of the material which a sampler is called upon to deal with, is, however, in a more or less divided state and fairly uniform. In practice it is assumed that 5 per cent, of the whole ( = 1/20th), if taken in portions of equal weight and at frequent and regular intervals, will represent the mass from which it was taken. Taking a heap of ore, A, and selecting one out of every twenty spade-, bag-, barrow-, or wagon-fuls, according to the quantity of stuff in the heap, there is obtained a second heap, B, containing one-twentieth of the stuff of the heap A. If we crush the stuff in B until this heap contains approximately the same number of stones as A did—which means, crushing every stone in B into about twenty pieces—B will become the counterpart of A. Selecting in the same manner 5 per cent, of B, there is got a third heap, C. This alternate reduction and pulverising must be carried on until a sample of suitable size is obtained. This may be expressed very clearly thus:—
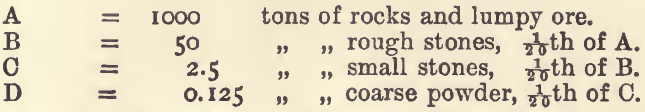
If the material to be sampled is already a dry powder, 5 per cent, of it should be heaped in a cone; each lot being added on
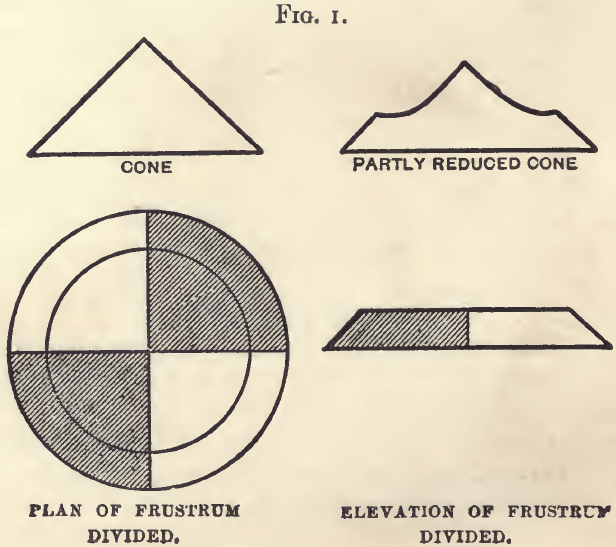
the apex of the cone already formed, so that it may distribute itself by falling evenly in all directions. When the cone is completed, convert it into a low frustrum of a cone, by drawing stuff uniformly and in a direct line from the centre to the circumference. Draw two diameters at right angles to each other, and reserving any two alternate quarters, reject the others. Mix; and form another cone, and proceed until a sample is got of the bulk required.
This is the usual plan, and all samples should be treated in this way when the stuff is fine enough to fall evenly down the sides of a cone.
Samples as they reach the assay office are seldom in a fit state for the work of the assayer; they are generally too coarse, and ought always to be more than he wants for any particular determination. The portion he requires should never be taken at hap-hazard; the sample must be reduced systematically to the quantity required.
1. If the sample is a liquid: it is sufficient to shake the bottle, and take out a measured or weighed quantity for the assay.
2. If a liquid with a solid in suspension: measure the whole of it. Filter. Make up the filtrate with the wash-water or water to the original bulk. Assay it. Dry and weigh the residue, and make a separate assay of it.
3. If of a creamy consistency, free from heavy particles: mix well; spread out evenly on a glazed tile. Take up equal portions at equal distances. Mix and assay.
4. If a mud of coarse and fine particles, or of particles of unequal density : weigh and transfer to a porcelain dish, or weigh in the dish. Dry at 100° C., weigh. Treat the residue as a solid capable of being powdered.
5. If a solid capable of being powdered, or already powdered: heap up into a cone; flatten with a spatula; divide along two diameters at right angles, and carefully reject the whole of two alternate quarters, brushing away any fine powder. Mix the other quarters, and repeat (if necessary). For small quantities a fine state of division is essential.
6. If a solid with metallic particles : powder and pass through a sieve; the metallic particles will not pass through. Weigh both portions and assay separately. Sifting should be followed by a very thorough mixing.
7. If a metal or alloy in bar or ingot: clean the upper surface of the bar, and bore through the bar. Use the borings. If the ingot or bar is small, cut it through and file the section. Filings must be freed from fragments of the file by means of a magnet; and from oil, if any be present, by washing with a suitable solvent. Where practicable, metals and alloys are best sampled by melting and granulating. The student must carefully avoid any chance of mixing dirt, or particles of other samples with the particular sample which he is preparing. One ore should be done at a time, and when finished, it should be labelled and wrapped up, or bottled, before starting on a fresh sample.
When an ore requires to be very finely ground in an agate mortar, it is often advisable to mix with a little, pure alcohol and rub until free from grit; dry at 1oo° C. and mix well before weighing.
When an assay is required of a quantity of ore made up of parcels of different weight and quality, each parcel should be separately sampled and parts of each sample, bearing to each other the same proportion by weight as the original parcels, should be taken and mixed. For example, a lot of ore is made up of one parcel of A, 570 tons, one of B, 180 tons, and another of C, 50 tons; a sample representing the whole may be got by mixing 57 parts of a sample of A with 18 parts of a sample of B, and 5 parts of a sample of C.
A bruising plate, like that in fig. 2, is convenient for general
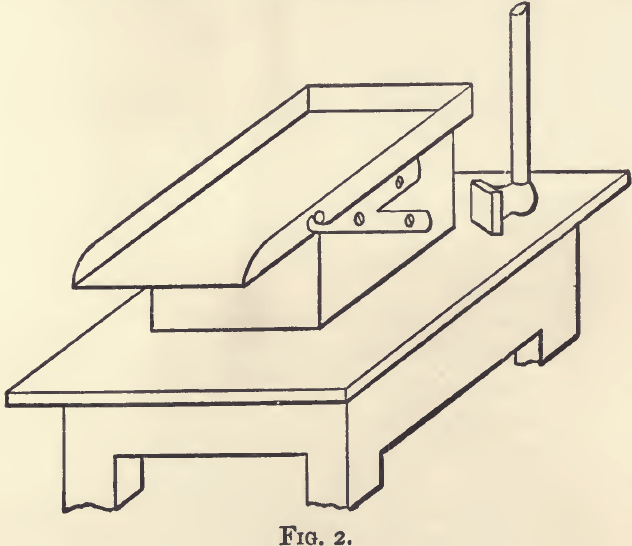
office work. The slab is of cast iron, about an inch thick. It is firmly supported on a solid block of wood, and pivoted for convenience in emptying. The bruising-hammer is steel-faced, about 4 inches square, and 1½ inch thick. The block is firmly fixed to a small table or tressel, so that the slab is about 2 feet 6 inches from the ground. The slab is cleaned, and the sample collected with the help of a stiff-haired brush.
