The final concentrate taken off the Wilfley table is not suitable for direct smelting. The “lines of separation’ as shown in fig. 14 (Part 5) are not always clearly defined as there is some intrusion of impurities, namely steel, into the concentrate zone and likewise some gold finds its way into the middlings. It is therefore necessary to include part of the middlings with the concentrate in order to prevent loss of too much coarse gold back into the circuit via the tailings launder.
Hence before the concentrate can be smelted it must be further upgraded. This may be done by purely mechanical means, e.g. re-runs over the Wilfley table, but such methods will invariably also result in the loss of some gold back into the circuit and also require additional labour.
It is preferable that all of the gold in the Wilfley concentrate be retrieved. Two traditional methods of achieving this are Amalgamation and Acid Digestion. Both methods have been practised throughout Australia for many decades and likewise both offer very good recovery if efficiently employed.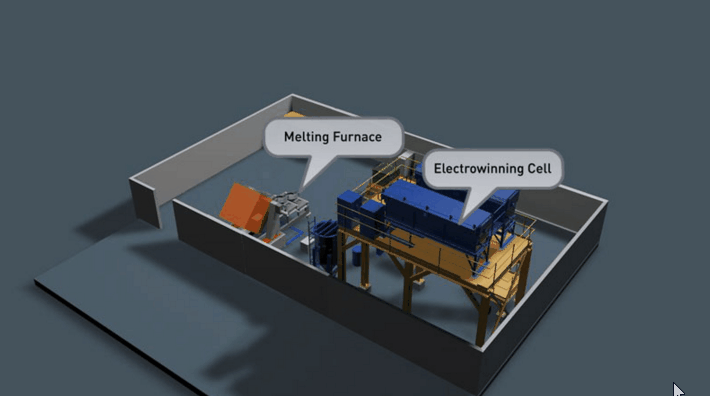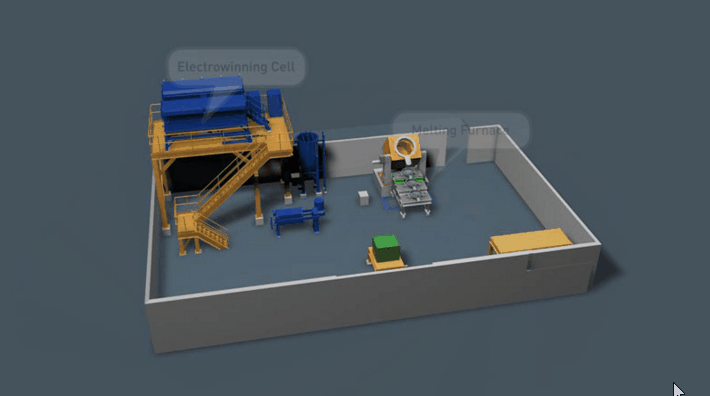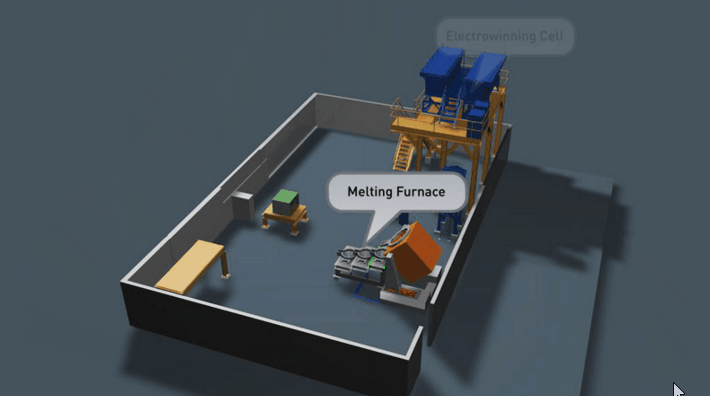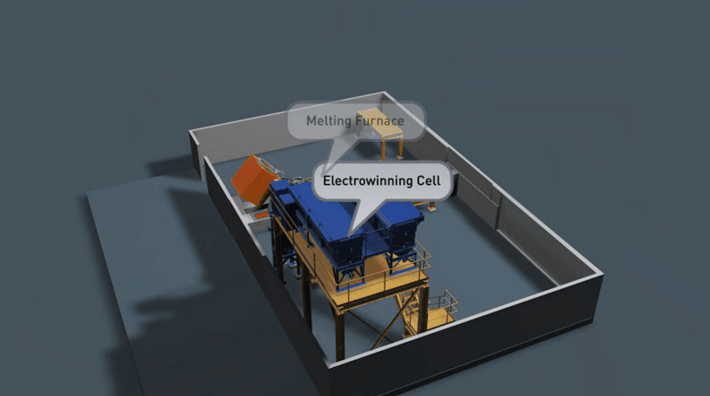
Amalgamation
This method involves the exposure of gravity concentrate to mercury in a rotating, fully sealed barrel. The mercury amalgamates with any free gold and this product is then separated from the remaining waste for later retorting. Retorting simply involves the heating of the amalgam so that the mercury vaporizes and passes into a condenser barrel leaving the gold “sponge’ behind.
This method is extremely efficient but in recent years the use of mercury with its associated danger has been frowned upon. Hence amalgamation has been rejected as a viable alternative here and for this reason it will not be covered in detail within this manual.
Acid Digestion
This method, as used here from the commencement of operations, involves the burning away or “digesting’ of impurities by exposing the concentrate to very strong acid solutions. If handled properly it can produce a very pure upgraded concentrate for smelting. It is however subject to censure on four accounts:
- It involves the use of highly dangerous acids.
- The Wilfley concentrate must be upgraded by mechanical means, i.e. as employed here the re-running of it over the Wilfley table, so as to get rid of some of the impurities and in so doing reduce the quantity of concentrate to an amount that may be efficiently treated by acid digestion.
- Acid digestion does present a security risk in that the daily upgraded product is of a very high quality and its form is such that some could be very easily “siphoned off’.
- Some loss of free gold may occur during the decanting off of acid and subsequent rinsing.

It is basically for the above reasons that the use of a more recent development “Intensive Cyanidation’ is being experimented with. However as acid digestion has been practised for so long here, a step by step procedure has been outlined below.
- Re-run the concentrate for a twenty four hour period over the Wilfley table in order to both upgrade the concentrate and in so doing reduce it to an amount of no more that 10 to 20 kilos.
- Expose this in a large container to hydrochloric acid using approximately two litres of acid to every kilogram of concentrate. Stir regularly.
- When the activity has ceased, decant off the acid, via another container, and then rinse the concentrate at least four times by filling the concentrate container with water and stirring; and decanting off each lot of rinse water.
- Put half a bucket of water into the concentrate container and then gradually introduce one bucket of nitric acid. Continual stirring is required during this stage and care must be taken to prevent frothing over and loss of concentrate.
- When the activity has died down, put the entire concentrate/acid solution mixture into the mechanically agitated vessel and leave it there for at least ninety minutes. This will ensure that all of the concentrate comes into contact with the nitric acid.
- Decant off the acid, again into another container.
- Rinse the concentrate thoroughly at least three times; decanting off each batch of rinse water into another container.
- Should there be any precipitate in the concentrate, this will indicate that not enough nitric acid was used. This can be easily broken down by exposing it to sulphuric acid. Use a 50/50 acid/water mix and put the water into the container first, Only a very small quantity of acid is required possibly no more than one to two litres at the most. After the precipitate has become entirely fluid, fill the container with water and stir well before decanting off the solution and rinsing the concentrate.
- Dry the concentrate and after recording the weight, lock it up in the strongroom.
Points to Watch
- All of the acids are extremely dangerous! Wear protective clothing and a full face mask fitted with a cartridge filter suitable for use in areas exposed to organic acid fumes.
- All of the digestion work must be carried out under the fume extraction hood.
- Make sure that the water to the scrubber has been turned on.
- Rinsing between the hydrochloric acid and nitric acid applications must be very thorough. The two acids when combined will dissolve gold and result in loss whilst decanting off.
- Any sediment from the containers decanted into must be returned to the concentrate container at each stage.
