In the process of discovering the nature of a rock and Identifying Minerals, the mineralogist may derive the necessary information by a careful study of its external appearance and characteristics; the form of crystallization, hardness, specific gravity, colour, streak (the colour when scratched, or when rubbed on a piece of porcelain), and also from its behaviour when exposed to the action of Chemicals or heat.
When examining a mineral specimen, the prospector is, to a great extent, guided by its colour. He may form a truer estímate by also noticing the lustre and streak. But it must be borne in mind that, for example, tinstone, though usually found of a brownish or blackish colour, is sometimes grey. Its streak, too, is not always brown, but sometimes grey & Cinnabar, too, is usually red, though occasionally brown or brownish-black.
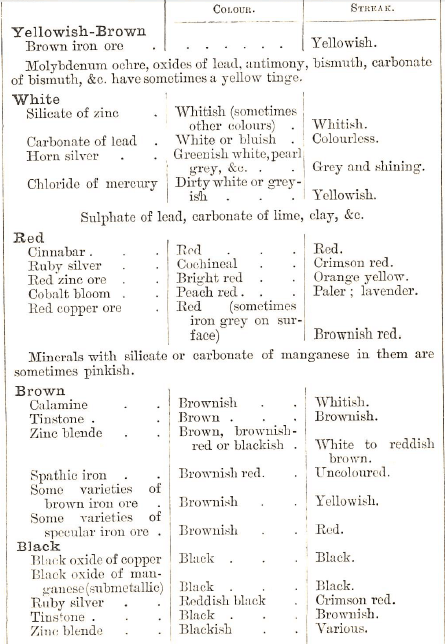
The following table may be of some use in the examination of some of the most commercially useful minerals:

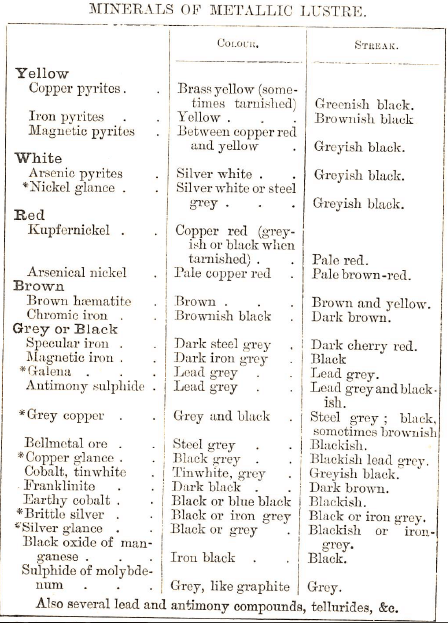
Certain micaceous iron minerals, which give a red streak, are some what metallic-like in appearance.

The speciíic gravity of a rock can often be approximately known by weighing it in the hand, and comparing it with an equal bulk of some other familiar rock ; but to accurately obtain the speciíic gravity of a mineral, a fragment of it should first be weighed in air, then in water (which can be done by suspending it to the scale of a balance and immersing it in water). The weight in air, divided by the weight in air minus the weight in water, gives the specific gravity.
S.G. = weight in air/(weight in air – weight in water)
But this method is more for the scientist than the ordinary prospector. The colour and appearance of the line or furrow on the surface of a mineral, when scratched or rubbed, is called the streak, which is best obtained by means of a hard tempered knife or a file. If the mineral is soft, it may be rubbed on a piece of rough porcelain. Those parts which have been much weathered should not be chosen.
To discover the hardness of a mineral, it is necessary to try and find out which of the typical specimens of the scale of hardness (commencing with the hardest and proceeding to the lowest) is scratched by it.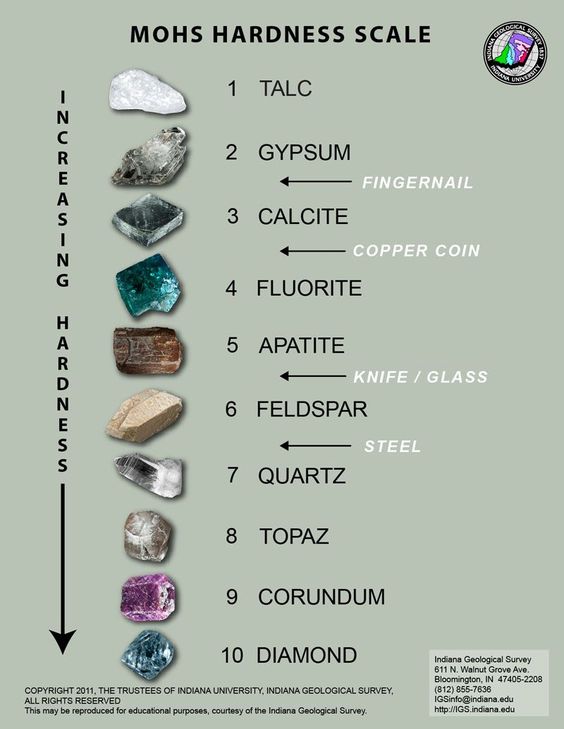
Mohs hardness scale definition
- Talc (such as soapstone), easily scratched by the finger nail.
- Rock salt (also gypsum, zinc), not easily scratched by the nail, nor can scratch a copper coin.
- Calc spar, calcite (transparent), both scratches and can be scratched by a copper coin, showing the hardness of both to be about equal.
- Fluorspar, not scratched by a copper coin and does not scratch glass.
- Apatite, with difficulty scratches glass and is easily scratched by a knife; scratches some kinds of glass, but not easily.
- Felspar, scratches glass and is not easily scratched by a knife.
- Quartz, not scratched by a knife and easily scratches glass.
- Topaz, harder than flint. (best done with smooth quartz crystal).
- Corundum, oriental emerald, sapphire, &c.
- Diamond, scratches any substance.
The hardness of minerals that can be scratched by the fingernail is 2½ or less, and by a copper coin loss than 4. Minerals may often be recognised, or their nature verified, by the crystallization they assume.

The exact degree of hardness can be found only when pure specimens of these ten minerals are at hand. In practice, one depends on the finger nail and the knife to get a rough approximation. Fine grained mixtures of minerals may be deceptive. For example, a little quartz, not noticeable to the eye, may cause a specimen of siderite to appear much harder than 3.5 to 4, the hardness of the pure mineral.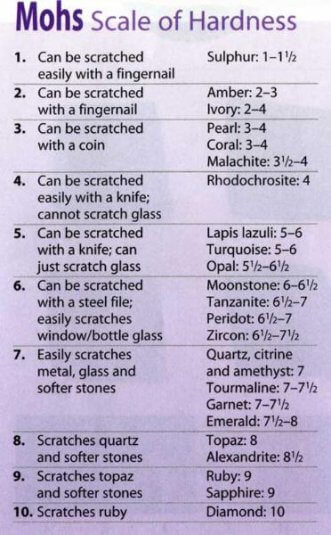
Testing for Hardness
When testing for hardness, care must be taken to note which object—the specimen or the standard—gives the line of powder when one is rubbed on the other. Thus, when a knife makes a scratch on apatite, the scratch can still be seen when the loose powder is rubbed off; but when a piece of feldspar is drawn over a quartz surface, the line it leaves can be rubbed off, and no scratch remains on the quartz, which indicates that the powder came from the feldspar and not from the quartz.
Judgment must also be used when testing specimens composed of small grains that are loosely held together. Each grain may be hard, and yet the specimen may give the false impression of being soft, due to the fact that the grains are easily separated from one another. For example, sandstone, which is made up mostly of grains of quartz, may sometimes be scratched by a knife, though each grain, if tested by itself, would be found to be harder than steel.
Hereafter, in stating the hardness of a mineral, the scale of hardness given in Art. 7 will be used, and the hardness will be denoted by H and a number. Thus, if the hardness be specified as H = 6, for example, this means that the mineral is of the same hardness as feldspar: and if specified as H=6.5, the hardness is between that of feldspar and that of quartz.
Identifying Minerals by Weight
When trying the weight of a piece of mineral in the hand, it is natural to think of its size; but its shape should also be noted; because what is really being done is to estimate the weight as compared with the bulk. A thin, flat specimen is apt to give the idea of largeness, while a thick or rounded piece of the same bulk looks smaller. That this simple rough test may give a reasonably correct idea of the weight as compared with the bulk, always choose a specimen that is as near as possible to a cube or a ball in shape. With practice and judgment, testing by trying in the hand will give a fair idea of the relative weight of minerals; it is quite easy, for example, to distinguish calcite, which is about 2¾ times as heavy as water, and barite, which is about 4½ times as heavy as water. Try this with specimens from your set of minerals. For exact work, the weight of a mineral is always compared with the weight of the same bulk of water, and the result is called the specific gravity of the substance, which will hereafter be denoted by G. Thus, the specific gravity of calcite is specified as G. = 2.7, which means that a piece of calcite weighs 2.7 times as much as the same bulk of water. The weight of a cubic foot of water is 62.4 pounds, and the weight of a cubic inch is 62.4 ÷ 1728=0.361 pound, hence, the weight of a cubic foot of calcite is 62.4×2.7 = 168.5 pounds, and the weight of a cubic inch is .0361 x 2.7 = .0975 pound.
How to Identify Minerals by Magnetism
If a mineral is attracted by a magnet it is said to be magnetic. The relative strength of this property (attraction) can be judged by noting the size (weight) of the pieces the magnet will pick up, because the specimen attracts the magnet just as much as the magnet attracts the specimen. If the strength of attraction be tested by powdering the mineral with the point of a knife, a wrong impression may be obtained on account of bits of steel being rubbed off the knife. It is therefore better to test grains of the mineral that are large enough to identify and separate.
Fundamental forms of crystals
1. Regular system (called the cubic, octahedral, &c.). In this system there are three equal axes (imaginary) passing through the same point and at right angles to each other. For examples—
2. Square prismatic system (has three axes at right angles to one another of which two are of equal length). Examples—
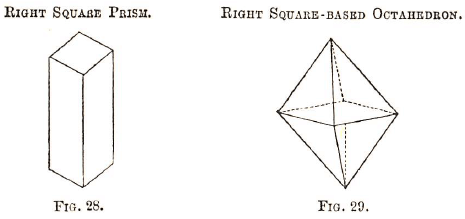
3. Right prismatic system (right rhomboidal or rectangular prismatic system), in which the three axes are of unequal length, though at right angles.
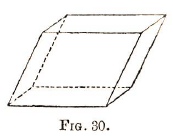
4. Oblique prismatic system, which includes the right rhomboidal prism and the oblique rhombic prism. The three axes may be of unequal length while two are at right angles, and the vertical axis inclined to one of these.
Example—Fig. 30.
5. Double oblique prismatic system in which three axes are unequal, and not any at right angles.
6. Rhombohedral system (regular hexagonal system). There are four axes, three of which are in the same plane and inclined to one another at an angle of 60°, the other being vertical: frequently the prism is capped by a six-sided pyramid.
Example—Fig. 31

Various names are given to the crystal mineral systems
- Cubic, regular system, monometric.
- Square prismatic, tetragonal, quadratic, dimetric.
- Right prismatic, rhombic, trimetric.
- Monoclinic.
- Triclinic or anorthic.
- Rhombohedral.
Crystalline form is not always sufficient evidence to rely upon in the determination of a mineral, as several different minerals assume the same or nearly the same shapes of crystal; and again certain particular minerals are found of more than one shape.
As examples of the first are carbonates of lime, lime and magnesia, zinc, iron, where the angle of the rhombohedral forms only vary between 105° and 108°. Sulphur, iron pyrites, specular iron, carbon, are examples of the second kind.
In addition to the already-mentioned characteristics useful in the determination of the nature of a particular mineral, some peculiar properties belonging to certain minerals should be noted.
For instance, some iron, cobalt, and nickel ores are attracted by the magnet; some minerals—such as fluor-spar, topaz, carbonate of lead, quartz, and calc spar—become electrified by friction ; others—such as calamine—become so when heated. Others, when rubbed, yield a peculiar odour; some—such as fluorspar—are phosphorescent, that is, yield a peculiar light when heated; while many possess a characteristic taste.
General Remark
Every prospector must have a thorough knowledge of minerals and their properties, the uses to which minerals are put, and the manner in which they occur; and he must be able to recognize them when he finds them. Although the number of minerals is very great, the prospector is concerned with only a small proportion of them; but, even so, the number is considerable, and the student must have some convenient way of getting information regarding them. For this reason, minerals have been divided into classes and groups, the minerals in each class having many properties in common.
The first step in identifying a mineral is to find to what class it belongs; this immediately separates that mineral from the minerals that belong to all other classes, and it greatly restricts the field of investigation. Then, by making certain simple tests, as described in the text, the various properties are revealed and the mineral is definitely identified.
It will be evident that the student must have a thorough knowledge of how to make these tests, and this can be acquired only by actual practice. By carefully studying the text and applying the instructions contained therein to the specimens it is recommended that he acquire, he will soon become proficient. In the beginning, it is advisable to work with specimens whose names he already knows, following the directions carefully, and comparing the results he obtains with the properties the specimen is known to possess. When he has become sufficiently familiar with the routine of the process, and has verified the statements in the text in connection with quite a number of different specimens, of all classes, he can then take a specimen he has found for himself, and can apply the same methods to its identification. By proceeding thoroughly and systematically in this manner, he will soon be able to identify minerals in the field.
While the student is not expected to memorize all that is here given relating to each separate mineral, it is advisable that he memorize everything possible regarding those of most importance or those in which he may be particularly interested.
Characteristics of Minerals
A mineral is a distinct substance that is found naturally in the earth’s crust; miners often use the word in the sense of ore or sometimes to apply to minerals, like pyrite, that accompany more valuable material, such as gold.
Minerals are usually more or less mixed, which is the reason for one difficulty in spotting (recogizing) them. Thus, quartz, when pure, is free from color, and it is like glass in appearance; but it is often found colored brown, red, pink, etc., due to small quantities of other substances mixed with it. The relative amount of the second substance that imparts the false color to the quartz may be very small.
Minerals can be spotted (recognized, identified) by such properties as color, weight, brittleness (or its opposite), malleability, crystal form, cleavage, etc. The student should read very carefully the following description of properties, and should then practise observing and testing these properties on specimens from the named set he can purchase, and comparing his results with the descriptions of the minerals in the text.
Color and streak
In addition to the difference caused by impurities, the color of a mineral varies more or less with the size of the grain, and this often gives a valuable method for determining the mineral. For example: coarse-grained hematite, which is of a shiny gray or black color, turns red when finely powdered (Try it with a small bit of specularite) ; a golden-yellow piece of iron pyrites gives a black powder; black zinc blende turns brown when rubbed to fine powder. In making this test, any means of getting a clean, fine powder of the mineral may be used; when the mineral is soft enough, this may be done with the point of a knife blade. Hold the knife short and shove it like a gouge, until a little powder gathers on the point. Rub this off on the palm of the hand or on a piece of white paper, and note the color. The color may often be seen on the specimen as it is rubbed. Hard minerals may be carefully rubbed on a surface of clean white quartz. Pieces of hard, unglazed porcelain are often used for this purpose, the piece of mineral being so rubbed on them as to make a mark or streak. The streak of a mineral is simply the fine powder made in this way. A very hard mineral can be powdered by rubbing or grinding one piece on another.
Luster
Luster is the appearance of the surface in regard to the way in which it reflects light. Metals have a peculiar luster, called metallic luster, and some minerals that are not metals have metallic luster. In the case of some other minerals, the luster is glassy, resinous, etc.
Hardness
The hardness of a mineral is tested by trying to scratch it with some common thing, such as the finger nail or a knife, or by rubbing one mineral against another. Use the knife as directed in Art.
