The subject here touched upon is one that can only be successfully handled by an expert chemist with a thorough training in both inorganic and organic chemistry; yet, as the metallurgical chemist may be asked to value a shale or petroleum, examine an oil for lubricating purposes or for the preservation of winding ropes—for these and other reasons this section has been inserted. Too frequently the metallurgical student’s training in organic chemistry is deficient, but it is hoped that he may be able to follow out the instructions given. That he may understand something of the composition of the materials examined and the reactions involved, he is recommended to peruse Remsen’s Elementary Organic Chemistry, or, if he intends following the subject further, he should, if possible, take up a systematic course of study—both theoretical and practical —in organic chemistry.
The methods here given are, at the best, somewhat patchy and brief, and suffer from the attempt to condense into a few pages matter which generally taxes the resources of a complete volume. The student therefore is recommended, on completing the tests here given, to consult Commercial Organic Analysis, vol. ii. part ii., Allen (aud Leffmann); also the technical methods of examining petroleum, lubricating and other oils, as given in Stillman’s Engineering Chemistry; also Lubrication and Lubricants, by Arch butt and Deeley.
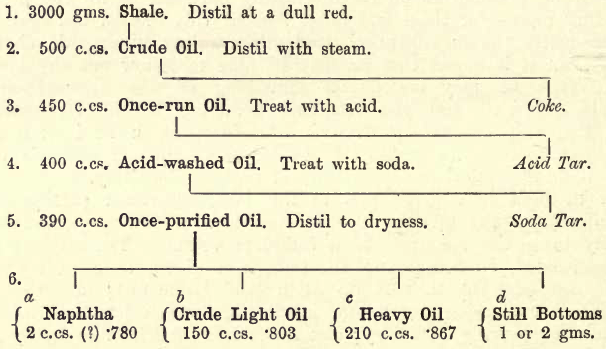
The following table given by Allen shows the nature and quantity of the products obtained from typical examples of American petroleum and Scotch shale oil. The student must remember, however, that considerable variations are met with in petroleums and shale oils, and that substances present in one may be absent in another. For instance, ‘kerosene’ from bituminous shale consists chiefly of ethenes (see Remsen), and from American petroleum largely of methanes.
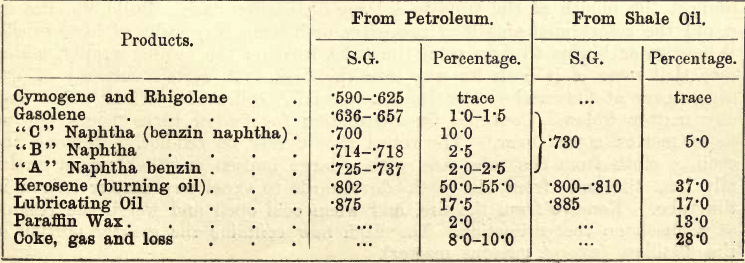
In the following partial examination of shale oil the student will see that the substances just detailed are not estimated singly but in four groups, in the ascending order of specific gravity .780, .803, .867, and residue over .867. For further separation of these groups consult Allen’s Organic Analysis.
EXAMINATION OF SHALE
An approximate idea of the nature of a shale may be obtained on examination by the method given for coals, but a more accurate idea of the commercial importance of its contents is obtained by the following method of ‘destructive distillation.’ (For the market value of shales, petroleum, etc., consult The Mineral Industry.)
The following tabulation (simplified and adapted from that given by R. Tervet in Allen’s Analysis) serves to give the student a bird’s-eye view of the various steps in the partial analysis.
The quantities of materials on the left are subject to considerable variation, and are inserted merely as an indication of what may be expected.
These products are not pure, but are mixtures. A certain amount of “naphtha” is mixed with the “crude light oil” and the “heavy oil.” Again, the greater part of the “heavy oil” consists of “lubricating oils,” and of the lesser part the bulk is “paraffin.” (See “Allen.”)
1. Details of the Analysis.—Take a 3000 gm. sample of the roughly powdered shale (10 sieve). Transfer to a large iron retort. Carefully lute on the lid with a paste of whiting. In a suitable furnace build a fire. Insert the retort and arrange a supply of cooling water for the tube, and connect the mouth of the tube to a large distillation flask. Build the fire up round the retort, enclosing it if necessary with temporary walls of loose bricks. When water begins to drip from the tube, turn on the cooling supply, taking care that none of it finds its way into the flask. A certain amount of gas comes over at first and is lost (unless specially collected). Then water and oily matter follow. Continue the distillation for two or three hours till no more matter comes over. The retort should now be red-hot. Remove the cooling cloth from the tube, and with a large bunsen or other means gradually heat the tube from the neck downwards to expel any matter lodged in the tube. Remove from the fire, and when cool open and weigh the residue as a check on the estimation. The flask now contains the greater portion of the distillate (except gaseous matter).
2. Distillation of the Crude Oil with Steam.—Arrange the distillation apparatus as in fig. 105. _
The flask on the left generates steam, which passes over into the distillation flask and aids the distillation of compounds boiling far above 100° C., the atmospheric pressure being divided between the water and oil, thus
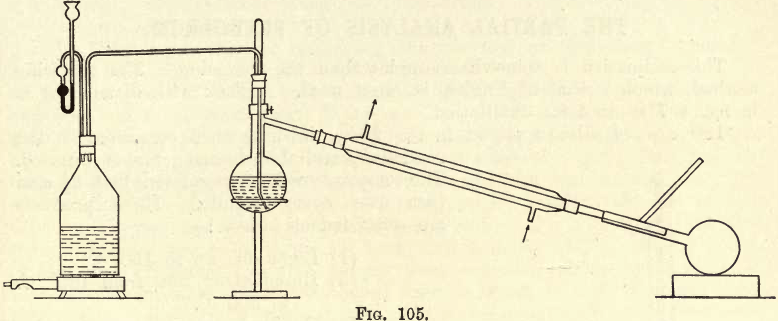
reducing the pressure on the oil, and obtaining the same result as by distillation in a partial vacuum. The mercury in the safety funnel acts as a safety valve.
Before proceeding with the distillation, take the specific gravity of the oil, either by the ordinary hydrometer, converting degrees Baume into the usual reading, and making the necessary corrections for temperature, or if only small quantities of the liquid are available, the Araeopicnometer of Eichhorn is very convenient.
The distillation is now commenced, the steam being first prepared, and then the heat is applied to the oil, through which the steam is slowly passed. Continue the distillation to dryness. The residue in the flask is “coke.”
3. Treatment of the “Once-Run Oil.”—The distillate in the receiver is transferred to a separator (fig. 106), and is warmed to about 40° C. by immersing the separator in warm water. Any water separating is run off. Then add 5% by volume of H2SO4 (S.G. 1.70) and agitate for ten minutes, plunging the separator into water at 35° C. to keep down the temperature. Allow to stand in the water for half an hour. Then run off the “ acid tar.”

4. Treatment of the “Acid-Washed Oil.”—To the contents of the separator add 20 c.cs. NaHO (S.G. 1.30). Warm to about 40° C. for half an hour and run off the “soda-tar.”
5. Treatment of the “ Once Purified Oil.”—Measure and take the specific gravity of the contents of the separator. Transfer to a clean distillation flask and proceed with the distillation. When the distillate reaches S.G. .780 replace the receiver by another. (Test the S.G. with a small Sprengel tube, the “ water contents ” of which are known. See “Allen.”) This first product is termed “naphtha,” and is only yielded by some oils. Measure the quantity obtained, if any.
Proceed again with the distillation till a drop of the distillate on a cold spatula shows signs of solidifying. Replace the receiver. The product thus obtained is “crude light oil.” Measure and take the S.G. of the quantity thus obtained.
Continue the distillation. If a viscid brown or yellow product appears, stop the distillation. Measure and take the S.G. of the distillate, which is termed “heavy oil” The residue in the flask is termed “still bottoms.”
From the volumes and S.G.’s of the products thus obtained, their percentages by weight may be calculated on the original sample of shale.
PARTIAL ANALYSIS OF PETROLEUM
This estimation is somewhat simpler than the foregoing. The following method, which is that of Engler, is often used. A flask with dimensions as in fig. 107 is used for distillation.
100 c.cs. of oil are placed in the flask, which is then connected to the condenser, and the thermometer is inserted.
The temperature is so regulated that 2½ c.cs. pass over every minute. Three products are separated as follows:—
(1) Light oils up to 150° C.
(2 Illuminating oils from 150° C. to 300° C.
(3) Residual matter.
Group (1) consists of cymogone, rhigolene, petroleum, ether, gasoline, naphtha, ligroin, and benzin.
Group (2) consists of varieties of kerosene.
Group (3) consists of lubricating oils, paraffin oils, and coke.
(For further details consult “Allen” and “ Stillman.”)
LUBRICATING OILS
The complete examination of lubricating oils involves much work and outlay for special apparatus. The following brief notes are given to enable the student to determine the acidity of an oil, its liability to oxidation, and other points which are of vital importance when an oil is used for fine machinery, or for the preservation of winding ropes (steel). In this latter case the lives of men often depend on the preservation of a rope, and it may be that a particular oil is used to protect it from acid waters, and that the oil itself may be as corrosive as the water. A low grade mineral oil may contain sulphuric acid which has been used for its purification, and has not been afterwards completely removed. Or again, an oil may contain organic acid, or, though not at the outset containing free acid, it may be so decomposed under certain circumstances that free acids are produced. The following points will therefore be considered here:—
(1) The Oxidisability of an Oil.
(2) The Acidity of an Oil
(a) As obtained from the manufacturer.
(b) After subjection to a high temperature in contact with water.
For determinations of viscosity, solidification, flash point, coefficient of friction, etc., consult the authorities previously mentioned.
(1) The Oxidisability of an Oil.—Most mineral oils and land animal oils are practically free from drying tendencies. Fish oils (except “sperm” and “ bottlenose ” oil) are less perfect in this respect. The vegetable oils differ considerably, some of them being far from perfect. This tendency to dry up is often termed ‘gumming.’
The following method of estimating the ‘drying’ or ‘gumming” qualities of oils is that of Fresenius, as modified by Bach.
Take a piece of 2 cm. bore soft glass tubing, closed at one end, and of sufficient length to hold about 200 c.cs. Draw it out to about 1 cm. bore near the other end. By means of an accurate pipette transfer 1 gm. of the oil to the tube. (Mark the pipette so that it delivers 1 gm. This may be done by delivering into a counterpoised watch glass on the balance pan. By a few trials the right point will soon be found.) Displace the air in the tube by oxygen, and seal up by the foot blowpipe the narrow part of the tube. Transfer the tube to an air bath, and heat at 110° C. for 10 hours (taking due precautions against danger from explosion of the tube). Allow to cool. In a beaker place 200 c.cs. of water. Break off the tip of the tube under the water, which then, if the oil has been oxidised, will enter the tube. Remove the tube with the water absorbed. Measure the residue in the beaker. The difference between this and 200 c.cs. represents the volume of oxygen absorbed. At the end of the experiment, test with a glowing splinter for free oxygen, and if none is present repeat the test with a larger tube or a smaller quantity of oil.
Mineral oils, as a rule, absorb very little oxygen; vegetable oils such as “rape,” “olive,” “cottonseed,” large quantities (for further data see “Allen”).
(2) The Acidity of an Oil.
(a) As obtained from, the manufacturer.
Mineral Acids (generally H2SO4) may be present as an accidental impurity. To test for such impurity, boil the oil with water for about 15 minutes. Separate the aqueous liquid. In one quarter of this liquid test for acid with methyl orange solution. A red colour indicates mineral acid. In another quarter test for the various acids with AgNO3, BaCl2 etc. In the remaining half estimate by the usual methods the acid found, if any.
Free Fatty Acids. —Warm 50 c.cs. alcohol (rect. spirit) to about 40° C., and add 10 drops of an alcoholic solution of phenolphthalein, and from a burette gradually add standard KHO (35 c.cs. = 5 c.cs. N. H2SO4) till a pink tint is obtained. Add 20 gms. of the oil to the solution. Keep the temperature about 40″ C., and agitate for about five minutes. If acid is present the pink disappears. Now gradually add from a burette more KHO till on agitation a permanent pink is obtained. The number of c.cs. of KHO taken in this last operation divided by 4 give the percentage of free fatty acid.
If free mineral acid has previously been found, it either must be allowed for or previously removed by repeated agitation with water.
(b) After subjection to a high temperature in contact with water (steam).
This determination is of importance, as oils are frequently used in contact with superheated steam, and under such conditions ‘ fatty oils’ are more or less decomposed into free fatty acids and glycerol. Such oils therefore should not be used for cylinders.
Heat 50 gms. oil + 50 gms. water in a strong bottle, with the stopper tied down, the bottle being immersed in boiling water. Agitate every now and then (taking precautions against explosion). After about 6 hours remove, and separate the aqueous liquid from the oil.
(a) The aqueous liquid.—Estimate the H2SO4 present. Any increase on that previously found is due to the decomposition of sulphonates (see Remsen), and if the increase be considerable, indicates an objectionable oil.
(b) The oil.—Titrate as before with KHO and phenolphthalein in an alcoholic solution. Any increase on the fatty acids previously found indicates decomposition of ‘fatty oils.’
Note.—In the case of cylinder oils for high-pressure cylinders, immerse the bottle in CaCl2 solution at 150° C. For general purposes, however, a temperature of 100° C. suffices to condemn or pass an oil.
