Silver ores, Silver Rocks and Minerals are easily fused before the blowpipe flame, either with or without carbonate of soda. The resulting globule of metal, of its characteristic white colour, can be readily hammered out or cut by a knife.
If the powdered mineral, supposed to contain silver, be dissolved in nitric acid and the solution be filtered or decanted, the presence of silver may be known by adding a solution of common table salt or of hydrochloric acid to the original solution. If silver be present, a white precipitate is thrown down. As chloride of lead or mercury might also be precipitated, let it be remembered that chloride of silver is soluble in ammonia, whereas chloride of lead is unchanged, and mercurous chloride blackened by it.
A very bright piece of copper, placed in the original solution, would be coated with metallic silver, if any existed. To test for copper, a bright knife-blade dipped into the solution would be coated with a copper film.
Sometimes, if a lump of silver-bearing ore be placed in a very hot fire, it will show white particles of the metal on the outside.
The silver metal soon tarnishes, when exposed to the action of sulphur ; thus, if boiled along with the yolk of an egg, it will blacken.
Native Silver
Found as wire silver, in thin sheets, in tree-like shapes, and as octahedral crystals.
Colour and Streak—silver white. When found in veins is usually tarnished on the surface.
Structure—easily cut and hammered out. H.—2·5 to 3; S.G.—10·1 to 11·1.
The silver usually contains gold and copper. Is recognised by the blowpipe and acids, as above mentioned. Native silver is often associated with iron rocks, native copper.
Brittle Silver Ore (sulphide of silver and antimony)
- Found massive, compact, in rhombic prism crystals.
- Lustre—metallic.
- Colour and Streak—black or iron grey.
- H.—2 to 2·5; S.G.—6·29.
- Composition—When pure contains about 71 per cent, of silver, the rest antimony.
With carbonate of soda before B.F. it decrepitates, but readily yields a silver lead. If the mineral be dissolved in nitric acid a piece of bright copper will be covered by a film of silver if placed in the solution. It is distinguished from silver glance by being brittle ; whereas silver glance is soft and sectile, and chips can be cut off without crumbling.
Silver Glance (sulphide of silver, argentite)
- A most important ore. Found massive.

- Crystallization—cubical, octahedral.
- Fracture—conchoidal or uneven.
- Colour— blackish or lead grey
(before exposure to the light has a bright metallic lustre). - Streak—same as colour, and shining.
- Structure—soft and sectile.
- H.—2 to 2·5; S.G.—7·1 to 7·4.
Contains 87 per cent, silver, the rest sulphur. Is usually associated with the sulphides of lead, copper, iron, zinc, antimony, arsenic, also with nickel and cobalt ores.
Before B.F. with carbonate of soda yields globule of metal. Known in acid solution by usual tests. Is similar in appearance to some copper and lead ores, but distinguished before the B.F. and by its malleability. Is fusible at the temperature of an ordinary flame.
Horn Silver (chloride of silver)
A soft mineral found massive; also in crystals. Is nearly opaque, translucent on the edges, and has a waxy appearance.
Fracture—conchoidal.
Colour—greenish white, pearl grey, brownish, dirty green, &c., and on exposure, brownish or purplish.
Streak—Shining and grey.
Can be cut like wax, the surface of the cut part being shiny.
Contains about 85 per cent, of silver, when pure. Not soluble in acids.
Fuses in a candle flame. Before B.F. readily yields metal. The surface of a plate of iron is silvered by it when moistened and rubbed. Occurs (often with carbonate of lead) in the upper parts of lodes. Silver bromide and iodide occasionally accompany the chloride. If a slice moistened be placed on a piece of zinc foil, the latter is soon stained black, and the chloride of silver partially reduced to metal on the surface against the foil. Forms a large portion of the South American “ pacos ” and “ Colorados” ores.
Ruby Silver (pyrargyrite)
- Massive, granular, or as prismatic crystals.

- Lustre—adamantine and submetallic.
- Colour—Sometimes black, reddish black, or brilliant cochineal colour.
- Streak—lovely crimson red.
- H.—2 to 2·5 ; S.G.—5·4 to 5·6.
Contains about 60 per cent, of silver, the rest arsenic. Occurs with calcite, galena. The dark red silver ore is a sulphide of silver and antimony ; the light red contains arsenic in the place of antimony.
The ores of silver occur in veins traversing granitic and gneissic rocks, clay slate, mica schist, limestone, and are usually associated with the ores of iron, copper, load (galena being always argentiferous), zinc.
At the rich mines of Leadville, Colorado, the silver is found in the carbonate of lead deposit lying between a blue limestone formation below and a white porphyry above (Fig. 41).
The famous Comstock lode, Nevada, consisting of quartz (here and there calcite and decomposed rocks), sulphides of various metals, silver ore as argentite, native silver, gold, lies between syenite above and metamorphic slaty rock below. In Mexico deposits of silver-bearing ore are found in limestone, also between slaty and porphyritic rocks, and traversing igneous and metamorphic formations. In Chili chloride of silver and native silver are found in stratified beds above granitic rocks, the richest belonging, it is supposed, to the Cretaceous period. In Peru are silver-bearing beds above porphyry, and with limestone at the sides. In Colorado and other Western States and Territories of America chloride of silver deposits occur in limestone, sandstone. Andesite, trachyte, rhyolite, are some of the country rocks in which silver lodes occur in South America, while the usual silver-bearing fissure veins are very numerous.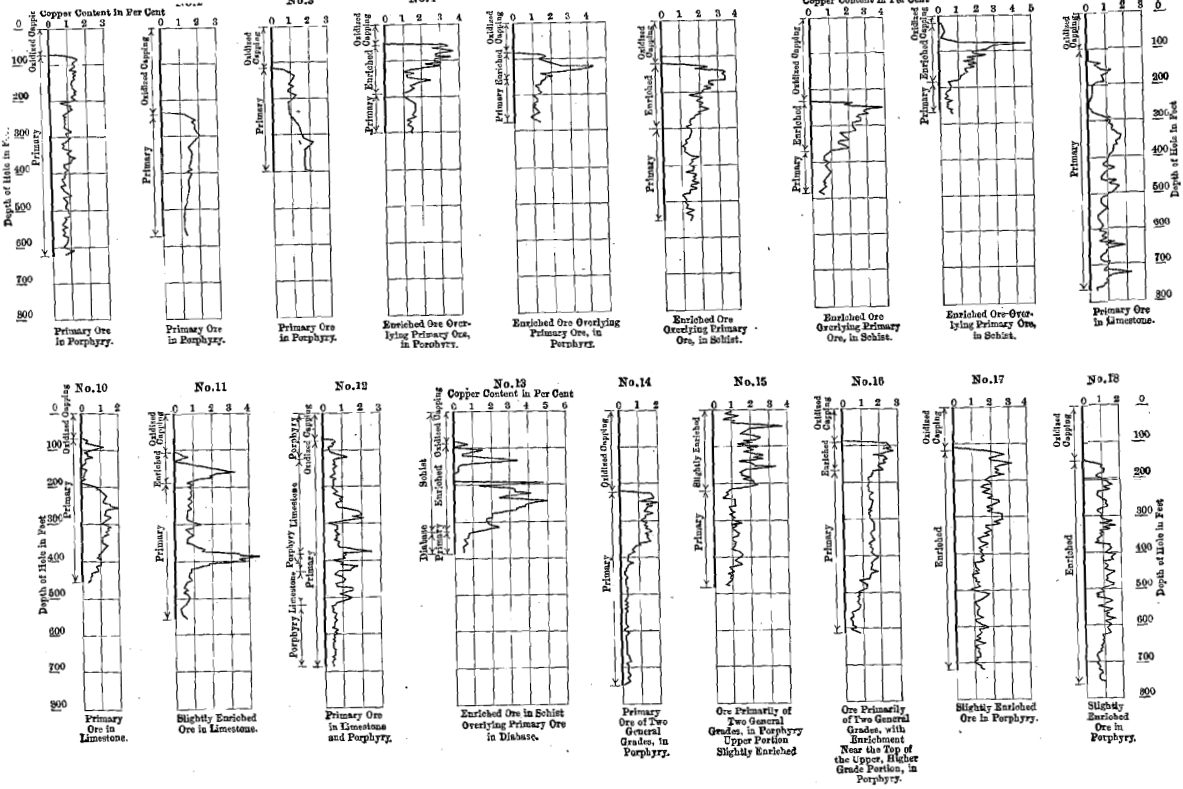
The silver mines of the Barrier Range, N.S.W., are in metamorphic rocks, chiefly mica schist. Near the surface the ore contains carbonites of lead and copper, chloride of silver, and, deeper down, sulphides. Manganese is sometimes present.
Of late years, the value of silver having fallen so very much, many mines—notably those with galena (sulphide of lead) veins—have had to be closed ; and whatever they were in the past, and unless silver rises in value, must be ranked as of too low a grade to be profitable; but this fact should not in any way make the prospector indifferent to any of the sulphide-bearing lodes. Whenever he notices an outcrop with traces of what would signify sulphides deeper in the lode, he should on no account rely on appearances, but should most assuredly have pieces of the rock properly assayed by an expert, because, for what he knows to the contrary, they may assay hundreds of ounces of silver to the ton, and perhaps contain gold as well. Several mines in Western America from which silver and gold used to be worked, are still worked chiefly for the gold rather than for the silver and gold.
As suggested before, the remembrance of chloride of silver and carbonate of lead (carrying silver), both of which occur in many silver-bearing lode districts, should retain a corner in the thoughts of the prospector, who, whenever he has the opportunity, should thoroughly examine, and even experiment upon, pieces of these so very easily passed by minerals. Considering that a pure piece of chloride of silver, sometimes found in lumps in New South Wales, Chili, &c., contains 75 per cent, of the metal, it is easily understood how valuable a deposit of the same may prove. The carbonate of lead, too (with silver), most unlikely by outward appearance to suggest value, often contains much of the valuable metal.
