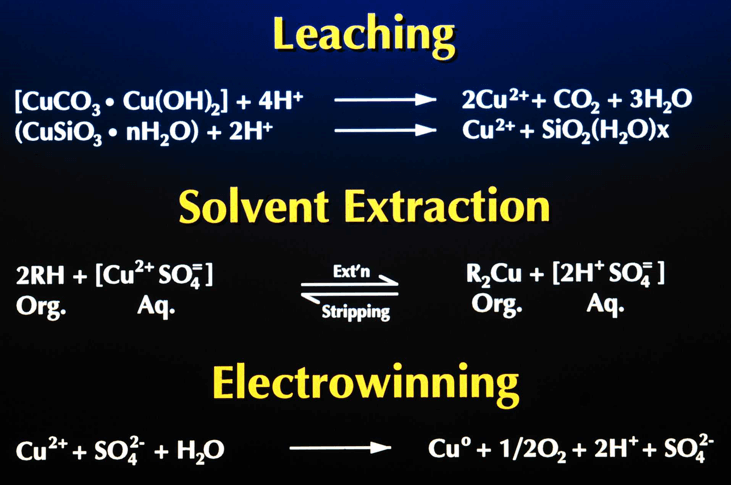
Solvent extraction, the process of extracting a specific metal valve from a solution to concentrate and purify that metal is a multistage process involving liquid ion exchange. In the first stage the metal value, in this example copper, exists in the aqueous leachate as an copper ion. The aqueous leachate is mixed with a organic solution containing a ion specific extractant. The extractants commonly used for copper extraction are chelating agents which form a copper-chelate complex in the acid conditions of the leachate. The solubility of this complex is controlled by the pH of the aqueous solution. At low acid concentration of the leachate the formation of the copper-chelate complex is favored. At higher concentrations the equilibrium reverses and the hydrogen ion-chelate complex is formed. The copper and hydrogen chelate complexes are in the organic phase. When the reaction proceeds in the copper-chelating forming direction the process is termed Extraction, the reverse reaction is termed Strip.
The extraction reaction reaction is represented as:
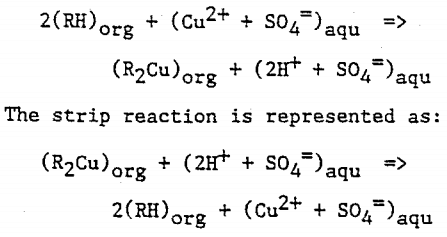
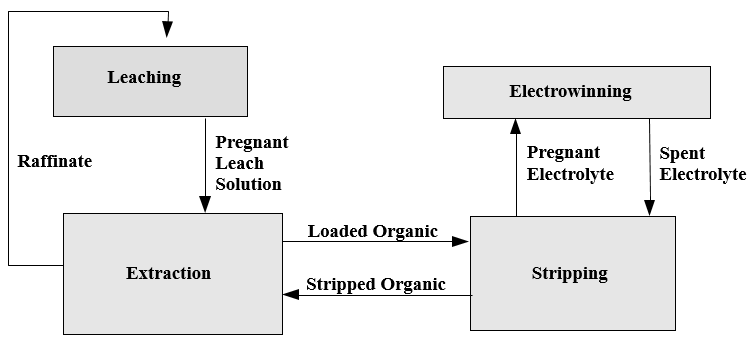
The extractant reagents are mixed with a diluent to form the organic phase. The diluent used in this process, normally kerosene, and the extractant are immiscible with the aqueous solution. This allows the separation of aqueous and organic phases. In the extraction process the aqueous phase, after the exchange of the copper ion for the hydrogen ion, is termed Raffinate. The organic phase is termed Loaded Organic.
The separation of phases and the adjustment of the pH allows the control of the location of the copper ion, in either the aqueous or the organic phases. The system described is more than solvent extraction, copper and hydrogen ions are exchanged between the organic and aqueous phases as controlled by the pH of the system. Hence, the term liquid ion exchange.
The reversible capability of the ion exchange reaction allows the controlled extraction of copper from the aqueous leachate into the organic phase. The subsequent reversal of the reaction allows the strip of the copper from the organic phase to a second aqueous phase. The reverse reaction is the basis for the strip stage. In this stage the organic phase containing the copper-chelate complex is mixed with a second aqueous phase. The greater acid concentration of second aqueous phase is sufficient to reverse the formation of the copper-chelate complex and favor the hydrogen ion-chelate complex formation. This transfers the copper to the aqueous phase and the hydrogen to the organic phase. In solvent extraction / electrowinning the second, acid rich , aqueous solution is the electrolyte from the tank house. This acid rich solution used to strip the copper from the organic phase is termed Spent Electrolyte. The aqueous phase after the second separation of the aqueous and organic phases, termed Strong Electrolyte, is the copper rich feed solution for the tank house. The copper depleted and acid enriched organic phase, termed Stripped Organic is returned to the extraction stage.
The chelating agents used in solvent extraction are designed to complex specific metal ions. This allows the selective separation of the copper in this example from the other acid soluble metals in the leachate. This preference is not at the total exclusion of other metals and therefor the selectivity of these reagents is very important to the successful application of the reagent. The selectivity of individual reagent is controlled by the pH of the aqueous solutions. Agents used in the solvent extraction of copper are generally cuprous (Cu²+) ion specific at pH 2 and less reactive with other metal ions. A change in the pH and the same reagent becomes specific for ferric iron (Fe³+) or other ions.
The structure of the chelate complex is a heterocyclic ring that includes a metal ion as one of its members. The molecular ring without the metal ion is commonly referred to as a claw and helps visualize the complex. This architecture of the ring gives the chelating agent its ability to form complexes with specific ions.
The chemical reactions given for extraction and strip shows that the system is stoichiometric. The ratio of chelate complexes to the metal ion is fixed. This requires that in the extraction process the concentration of reagent in the organic phase be determined by the concentration of the metal ion in the aqueous phase. If excess reagent is available after the complexing of the copper from the aqueous phase, the excess reagent will complex with the next preferred metal ion. If excess copper remains in the aqueous phase after all of the reagent has formed the copper- chelate complex, the copper will remain in the aqueous phase. Copper will be returned to the well field in the raffinate, but this preferable to extracting iron into the organic phase and ultimately transferring it to the electrolyte.
A second result of the stoichiometric nature of the reactions is the increase in the concentration of copper from the leachate solution to the strong electrolyte. The volumes of the aqueous and organic phases do not change when the ions are exchanged between the phases. The concentration of copper in the electrolyte is the same concentration as that of the hydrogen in the spent electrolyte when adjusted for molecular weight. Because the acid strength of the strip solution is required to be high, the concentration of the copper in the aqueous phase, after the exchange of the copper and hydrogen ion, will be high.
Solvent extraction as described in this simplified manner produces a high quality electrolyte for electrowinning of copper. The selective extraction of copper from the leachate and the concentration of the copper yield a pure, copper rich electrolyte. A generic electrolyte from solvent extraction would be an aqueous solution with approximately 150 grams per liter acid and a copper tenor of 30 – 45 grams per liter.
Key Facts About Copper Solvent Extraction
- First plant in 1968, now practiced on 6 continents
- Most plants produce LME quality copper
- Small (~ 1000 T / yr) to large.
- Very simple (1 train at 2E,1S leaching one type ore)
- Complex (4 trains at 2E,2E,1W,1S leaching antacamite and chalcocite)
- Very complex (4 SX plants, 3 tankhouses, dump leach, heap leach, concentrate leach, oxide and sulfide ores)
- Six types of leaching
Heap, dump, in situ, vat, agitation, autoclave
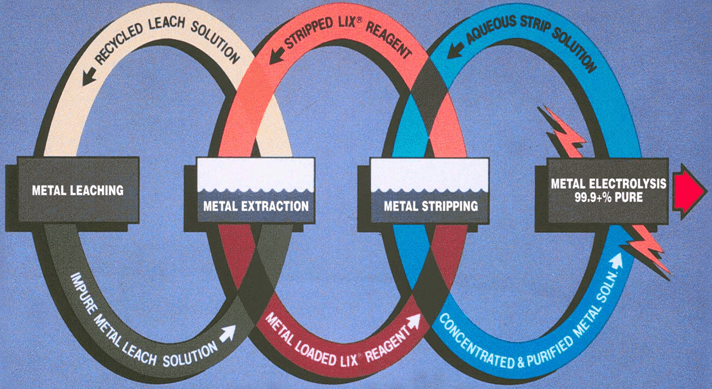
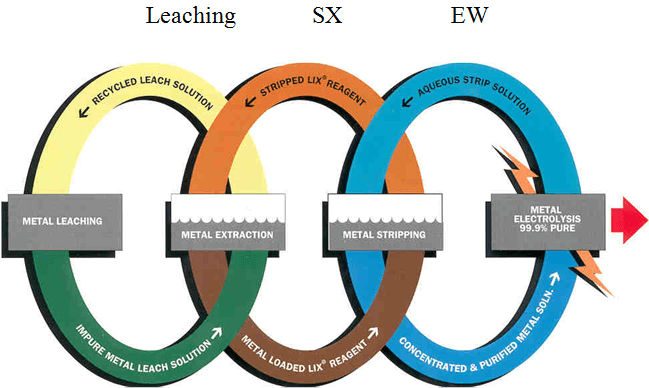
Copper Recovery Process Composed of 3 Individual Processes
Leaching
– Puts copper in an acid water solution
Solvent Extraction
– Transfer copper from leach to electrowinning
– Transfer acid from electrowinning to leach
Electrowinning
– Transfers copper from solution to metal
The Process of Leach/SX/EW

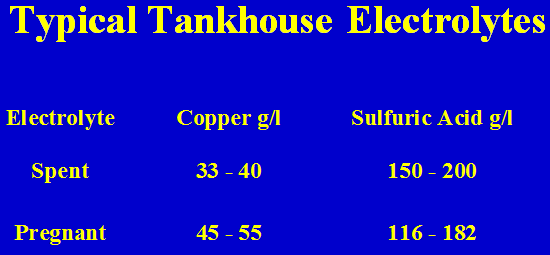
Typical Sulphuric Acid Leach Solutions
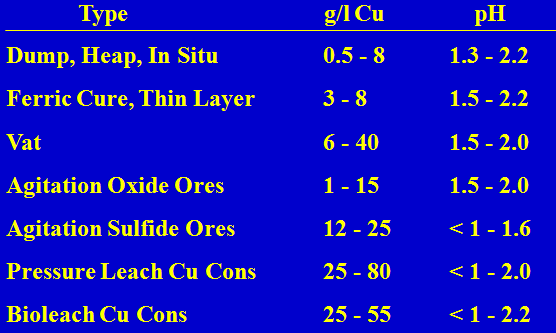
Common Features: Fe2+, Fe3+, sulfate salts of many metals including Mn, Mo and U at times
Typical Tankhouse Electrolytes
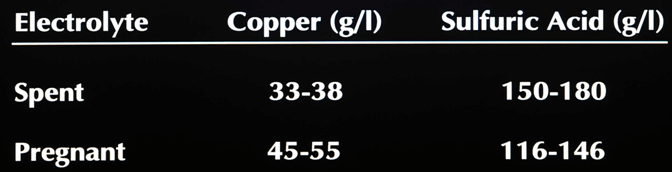
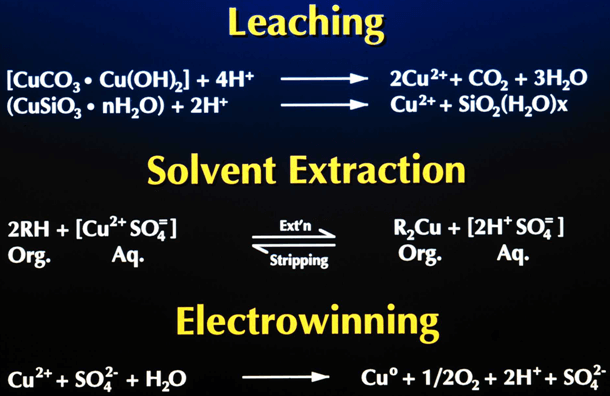
Process Description
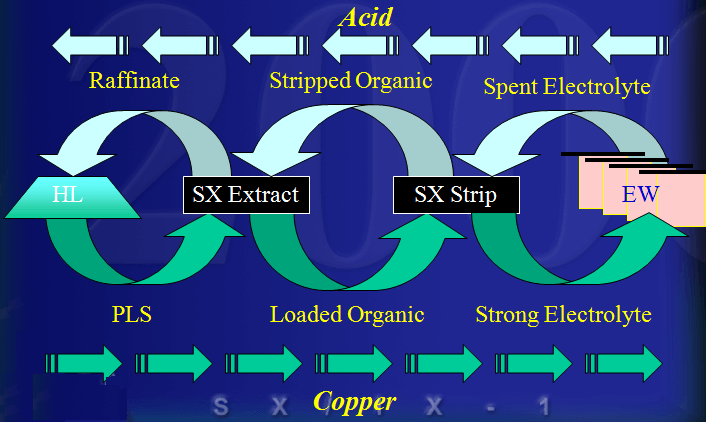
Objectives of Solvent Extraction
Purification of copper
–Extract copper, leave other metals behind
Concentration of copper
–In most cases from under 10 g/L up to 55 g/L
Conversion from one anion to another
–Chloride to sulfate
–Ammonia to acid sulfate
Agitation Leach Plants are Normally the Most Difficult L/SX/EW Plants to Run
- Solids in the leach solution
- Chemicals used in solids / liquid separation
- Variable copper concentration in leach solution

Solids in the Leach Solution
SX plants handle solids better if mixers are organic continuous
–Crud goes to the interface
Solids flip mixers to aqueous continuous
–Crud floats and moves to next mixer
Unstable plant or maybe a crud run
Solids cause crud generation
–Reduce flow capacity of plant, maybe a plant shutdown
–Crud removal is a labor intensive unpleasant job
–Organic losses are high in crud
Solids in electrolyte
–Rough cathodes
–Impurities in cathodes
–Carries organic to the tankhouse
Cathodes stick to blanks
Organic on cathode
Chemicals used in solids/liquid separation–Flocculants and Coagulants
- Use too little gives solids in leach solution
- Use too much and these chemicals are in the leach solution along with solids
–Gives lots of sticky crud that may float and move or it may form a gelatinous mess that is very difficult to remove and treat
Running the CCD circuit properly to flocculate the solids without using too much flocculant is a very important job!

Variable Copper Concentration in Pregnant Leach Solution
- Impact Production
- Impact Selectivity
- Operating parameters must be adjusted to optimize plant performance.
-Reagent Concentration
-Advance O/A ratios (flow rates) - Why does this make running a copper solvent extraction plant more difficult?
-Reagent concentration is designed for a certain copper concentration at the plant design flow
-Reagent concentration can be easily increased but not easily decreased
-Copper not extracted is partially lost to tailings
-Too much reagent raises costs and increases impurity transfer
-Not transferring enough copper reduces production
-Transferring too much copper may end up precipitating copper sulfate in pipelines, settlers, filters, etc.
What Should be Done
If possible blend ore from mine to achieve a more constant grade
Use a reagent that gives good performance over a range of conditions, LIX 984N
Use a reagent concentration designed to give the desired copper transfer at design organic flow
If increase in copper in the PLS is for the short term increase organic flow
-Must have enough organic in the surge tank to handle increased organic flow
-Most mixer settlers can treat a flow well above design, especially for a short time
If increase in copper in the PLS is for the long term increase reagent concentration
If copper in PLS decreases in the short term increase PLS flow and/or decrease organic flow.
If copper in the PLS decreases for the long term decrease reagent concentration
Calculating Correct Flows
Mass Balance Calculations
Copper cannot be created or destroyed in a leach/solvent extraction/electrowinning operation. It can only be transformed and/or transferred.
When copper is leached is the copper transformed or transferred or both transformed and transferred?
In a solvent extraction step is the copper transformed or transferred or both transformed and transferred?
When copper is plated in an electrowinning operation is the copper transformed or transferred or both transformed and transferred?
Therefore copper leached must over time equal copper produced plus copper lost to tails.
The Process of Leach/SX/EW
Mass Balance – Leaching
The mass of copper leached from the ore in a given period of time is equal to the increase in the mass of copper gained by the leach solution in that same period of time.
Example: 10,000 MT ore at 1.2 % copper agitation leached/day at 96 % leaching efficiency and 98% washing efficiency. Flow of leach solution is 750 m³ / hour off the first stage of CCD. Copper in raffinate entering CCD circuit is 0.44 g/l Cu. What is the copper concentration in the leach solution?
Cu leached = 10,000 MT ore/day x .012 MT Cu/MT ore x 0.96 = 115.2 MT/day
115.2 MT Cu x 0.98 = 112.9 MT Cu in leach solution/day
112.9 MT/day/24 hour/day = 4.704 MT/hour
Copper gained by leach solution
[(4.704 MT/hour) (1000 kg/MT) / (750 m³/hour)] = 6.27 kg/m³ (g/l)
Copper concentration in leach solution = copper in raffinate + copper gained
= 0.44 g/l + 6.27 g/l = 6.71 g/l
Mass Balance – Extraction
In the extraction section of solvent extraction the mass of copper lost from the leach solution in a period of time is equal to mass of copper gained by the organic phase in that same period of time.
Example: 750 m³ / hour of PLS having 6.71 g/l Cu is treated in a copper SX plant with 2 extraction stages and 2 strip stages with an organic solution containing 20 volume % LIX 984N flowing at 800 m³ / hour. The stripped organic contains 2.6 g/l Cu and the copper recovery is 93.44 %. What is the copper concentration on the loaded organic?
Copper lost by aqueous = copper gained by organic
750 m³/hour x (6.71 kg / m³) (0.9344) = 4702.5 kg/hour lost by aqueous
Copper gained by organic = 4702.5 kg/hour / 800 m³/hour = 5.878 kg/m³
Copper on loaded organic = copper on S.O. plus copper gained =2.6 g/l Cu + 5.878 g/l Cu = 8.478 g/l Cu
Mass Balance – Stripping
In the strip section the mass of copper lost from the organic phase in a period of time is equal to the copper gained by the electrolyte phase in that same period of time.
Example: The barren electrolyte has 35 g/l Cu and 189.3 g/l acid while the pregnant electrolyte has 50 g/l Cu. What is the flow rate of the electrolyte?
Copper lost by organic in stripping = copper gained by electrolyte in the same period of time.
800 m³ / hour (loaded organic – stripped organic) = copper lost by organic
800 m³ / hour ( 8.478 kg Cu / m³ – 2.60 kg Cu / m³) = 4702.5 kg Cu / hour
4702.5 kg Cu / hour / by gain in copper by electrolyte = flow electrolyte
4702.5 kg Cu / hour /(50 kg Cu / m³ – 35 kg Cu / m³) = 313.5 m³/hour
What is the copper in the pregnant electrolyte if the electrolyte flow drops to 300 m³ / hour?
Mass Balance – Electrowinning
In EW the mass of copper plated in a given period of time is equal to the mass of copper lost from the total electrolyte volume in that same period of time.
Example: If the barren electrolyte has a copper concentration of 35 kg Cu / m³ and the pregnant electrolyte has a copper concentration of 50 kg Cu / m³ at a flow of 313.5 m³ / hour for 24 hours how much copper is plated?
Copper plated in a period of time = copper lost from electrolyte in the same period of time.
Copper lost from electrolyte = (313.5 m³ / hour)(24 hr /day) (15 kg Cu / m³) = 112,900 kg Cu / day = 112.9 MT Cu / day
Mass Balances
A mass balance can be done for the entire circuit or for a single stage
Example: Circuit modeling using program on 2E, 2S with the feed, electrolytes, organic and recovery of preceding pages gives following data.
PLS = 6.71 g/l Cu
Extraction stage 1: Organic = 8.482 g/l Cu Aqueous = 2.518 g/l Cu
Extraction stage 2: Organic = 4.551 g/l Cu Aqueous = 0.436 g/l Cu
Strip stage 2: Organic = 2.60 g/l Cu Aqueous = 37.6 g/l Cu
Strip stage 1: Organic = 3.61 g/l Cu Aqueous = 50.0 g/l Cu
O/A extraction = 800 / 750 = 1.06667
O/A stripping = 2.556
Mass Balances for Acid in a Copper SX Circuit Cu Extraction/Stripping Equilibrium
![]()
When one Cu2+ is extracted one molecule of H2SO4 is created
When one molecule of H2SO4 strips organic one Cu2+ is liberated
The mass of H2SO4 is 98 amu and the mass of Cu is 63.54 amu.
Thus 1.54 mass units of sulfuric acid equals 1 mass unit of Cu.
Said in another way 1.54 grams of acid = 1 gram of Cu across SX
Electrowinning Reactions
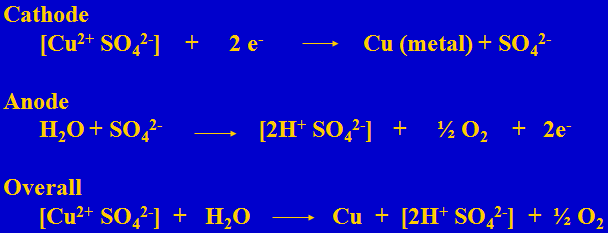
In electrowinning one Cu is plated and one sulfuric acid is generated. Thus 1.54 grams of acid is generated when one gram of copper is plated.
Copper and Acid Mass Balances in a Copper Leach/SX/EW Plant
For every gram of copper extracted from the leach solution 1.54 grams of acid is added to the raffinate
For every gram of copper stripped from the loaded organic 1.54 grams of acid is lost from the electrolyte
For every gram of copper plated in the tankhouse 1.54 grams of acid are produced
Example: What is the acid in our pregnant electrolyte?
-BE: 35 g/l Cu, 189.3 g/l acid
-PE: 50 g/l Cu, 166.2 g/l acid
-Calculation: [189.3 g/l acid– (15 g/l Cu x 1.54 g/l acid/g/l Cu)]
Why Are Mass Balances Important
- Give information on flowmeters
- Give information on mixer efficiencies
- Give information on excessive entrainment
- Used to detect theft
- Used to optimize leaching economics
- Used to detect leaks
- Others??
Determination of the Advance O/A Ratios
- From flowmeters. Organic flowmeters often not accurate
- Sample the mixers. Depends on where the mixer is sampled
- From copper analysis. Usually the most accurate
Example Extraction:
[g/l Cu(PLS) – g/l Cu(raf)] x PLS flow = [g/l Cu L.O. – g/l Cu B.O.] x Organic flow
Advance O/A extraction = Organic flow / PLS flow = [g/l Cu(PLS) – g/l Cu(raf)] / [g/l Cu(L.O.) – g/l Cu(B.O.)]
[ 6.71 – 0.44 ] /[ 8.48 – 2.60 ] = 6.27 / 5.88 = 1.066
Example Stripping:
Advance O/A stripping = Organic flow / Electrolyte flow = [g/l Cu(P.E.) – g/l Cu(B.E.) / [g/l Cu(L/O.) – g/l Cu(B.O.)
[ 50 – 35 ] / [ 8.48 – 2.60 ] = 15 / 5.88 = 2.56
Mixer Efficiency
Mixer Efficiency is defined as the copper transfer in a mixer / the copper transfer at equilibrium. For extraction stage 1
1. Analyze organic and aqueous phase entering a mixer
2. Sample organic and aqueous phases exiting mixer then analyze for Cu.
3. Mix organic and aqueous phases entering the mixer at the advance O/A ratio then shake vigorously for 10 minutes. Analyze each phase for Cu. (equilibrium values)
Calculations for extraction stage 1.
From organic analysis
E1 mixer org g/l Cu – E2 org g/l Cu x 100 = Mixer Efficiency (Org)/Equil E1 org g/l Cu – E2 org g/l Cu
From aqueous analysis
PLS g/l Cu – E1 mixer aq g/l Cu x 100 = Mixer Efficiency (Aq)/PLS g/l Cu – Equil E1 aq g/l Cu

Stage Efficiency
Stage Efficiency is defined as the copper transfer in a stage/the copper transfer at equilibrium. For extraction stage 1
1. Analyze organic and aqueous phase entering E1mixer
2. Sample organic and aqueous phases exiting E1settler then analyze for Cu.
3. Mix organic and aqueous phases entering the E1mixer at the advance O/A ratio then shake vigorously for 10 minutes. Analyze each phase for Cu. (equilibrium values)
Calculations for extraction stage 1.
From organic analysis
E1 org weir g/l Cu – E2 org weir g/l Cu x 100 / (Org) = Stage Efficiency
Equil E1 org g/l Cu – E2 org weir g/l Cu
From aqueous analysis
PLS g/l Cu – E1 aq weir g/l Cu x 100 = Stage Efficiency (Aq)
PLS g/l Cu – Equil E1 aq g/l Cu
Mixer Efficiency and Stage Efficiency
Mixer efficiency from organic analysis should be very close to mixer efficiency from aqueous analysis.
Stage efficiency from organic analysis should be very close to stage efficiency from aqueous analysis.
Mixer efficiency should be equal to or only slightly less than stage efficiency.
Most people do not distinguish between mixer efficiency and stage efficiency.
Why is Mixer / Stage Efficiency Important?
Poor mixer efficiency gives poor metal recovery, i.e., metal transfer must take place in the mixer or it will not take place at all.
Poor mixer efficiency may point out a problem with mixing equipment.
Poor mixer efficiency may point out a problem with contamination of plant organic.
Typical mixer efficiency values
92% ± 2% extraction stage 1
94% ± 2% extraction stage 2
97% ± 2% extraction stage 3 or parallel stage of extraction
> 98% stripping stages
Net Copper Transfer on the Organic
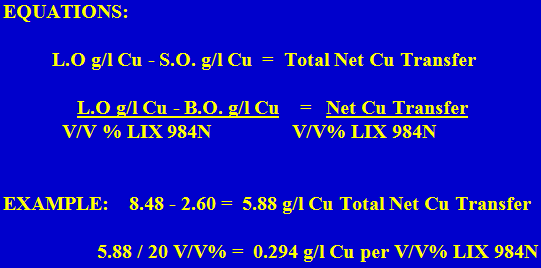
Net transfer numbers can be used to adjust the reagent concentration or the advance O/A ratios to compensate for fluctuations in the PLS Cu grade.
Net Transfer per V/V% LIX 984N used to adjust Reagent Concentration:
PLS Grade Increase: The PLS Grade increases from 6.71 g/l Cu to 7.05 g/l Cu? This represents an increase of 0.34 g/l Cu.
EQUATION:
g/l Cu increase in PLS grade/Net transfer V/V% (0.294 g/l Cu) = Increase in Vol% reagent needed
EXAMPLE:
0.34 g/l Cu/0.294 g/l Cu = 1.16 v/v% more LIX 984N needed
Net Cu transfer value used to adjust Advance O/A ratios
PLS Grade Increase: The PLS Grade increases from 6.71 g/l Cu to 7.05 g/l Cu. This represents an increase of 0.34 g/l Cu.
Note: Total Net Transfer = L.O. – B.O or 8.48 – 2.60 = 5.88 g/l Cu
Equation To Adjust the Organic flow
Org Flow (Total Net Transfer + g /l Cu increase in PLS )/Total Net Transfer = New Org Flow Rate
EXAMPLE:
800 m³ / hour (5.88 +0.34 )/5.88 = 846 m³ / hour (New Org Flow Rate)
Best Way to Handle Increase in Copper Concentration of PLS?
When do you increase reagent concentration?
If change in PLS copper concentration is long term
If organic flow cannot be increased
If O/A ratio far away from 1/1 and mixer efficiencies affected
When do you increase organic flow rate?
If change in PLS copper concentration is short term
If organic flow rate is not at maximum and O/A ratio is near 1
If you have no reagent to increase reagent concentration
LIX 984N Concentration Determination
Under the conditions outlined in the Quality Control Procedures the maximum copper loading capacity for each v/v% of LIX 984N is 0.53 g/l Cu, so 10.0 V/V% loads 5.30 g/l Cu.
Reagent Concentration = Organic Max Load g/l Cu/0.53 g/l Cu
Example: 8.48 g/l Cu / 0.53 g/L Cu / Vol% = 20.0 V/V% LIX 984N
What Will Cause a Poor Mass Balance
- Inaccurate Flow Measurements
- Inaccurate Analyses
- Inaccurate Sampling
- Inaccurate Calculations
- High Entrainments (Aq in Org)
Electrowinning
Electrowinning
- Electrowinning Reactions
- Cathode Quality
- Electrolyte Quality
- Solvent Extraction
Electrowinning Reactions
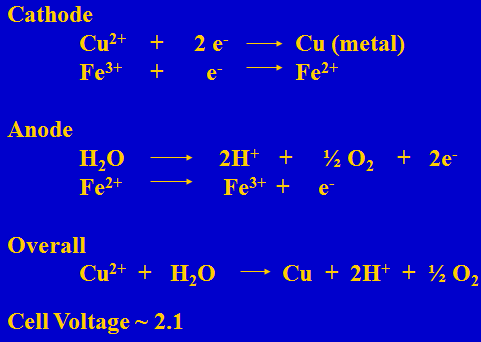
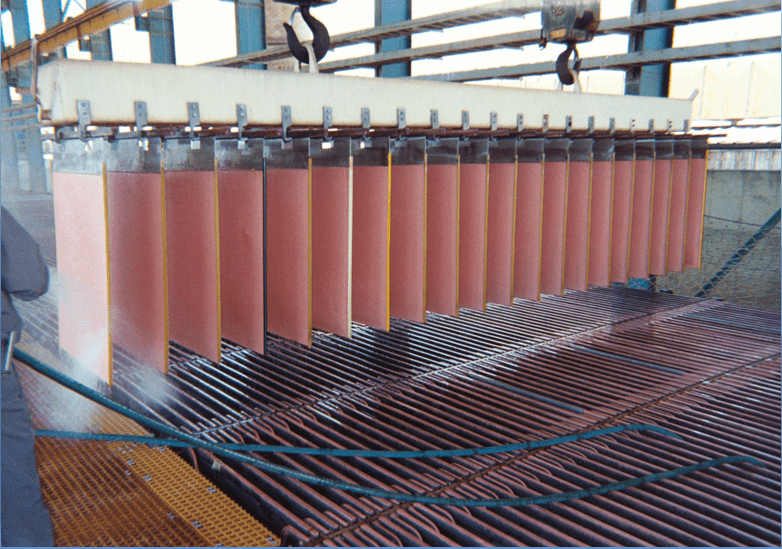
Cathode Quality
- Impurities occluded in the cathode
-PbSO4, PbO2, Silica, solids carried to tankhouse, electrolyte, organic - Organic burn caused by circuit organic in tankhouse
-Copper SX reagent enhances porous copper burn - Rope around edge of cathode
-Can be porous
-smaller anode than cathode

High Purity Cathode
- Promote smooth, dense deposit
- Stable lead anode
- Clean electrolyte
- Prevent direct contact of anode and cathode
- Wash cathodes effectively
Promote Smooth Dense Deposit
- Consistent anode-cathode spacing & alignment
- Lowest practical current density
-Design 260 to 280, run 260 to 340 amps / m² - Highest practical copper concentration
-Electrolyte entering cell (35 to 41 g/l Cu)
-Good electrolyte circulation in cell (3 g/l Cu drop) - Temperature – 45 to 50 °C
- Smoothing agent – EW guar, polyacrylamide
- Excellent house keeping
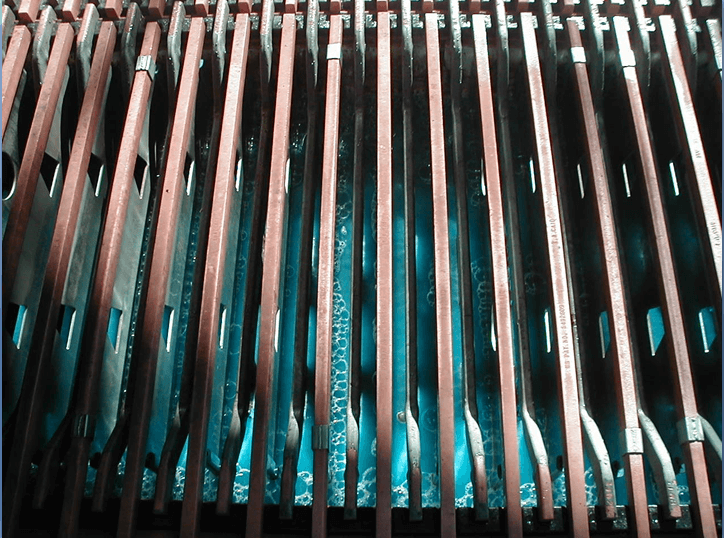








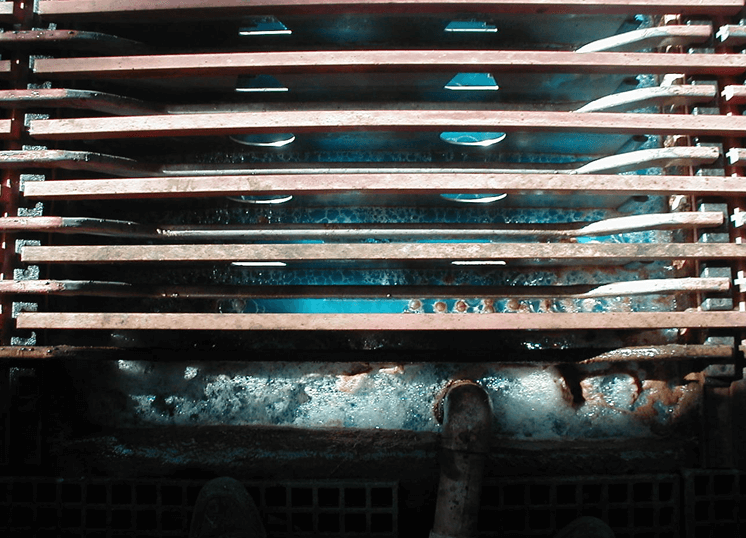
Electrolyte Quality
- SX provides high copper concentration
- SX can provide good chemical purity
-Reagents very selective for copper
-Entrainment of aqueous in loaded organic - Remove organic entrainment from electrolyte
-settling, flotation, filtration - Remove solids from electrolyte
-settling in cell, filtration

Stability of Lead Anode
- Formation of lead oxide on anode surface
-Choice of lead alloys
-cobalt in electrolyte
-manganese in electrolyte
-Maintain power to the tankhouse - Physical stability
-Cast or rolled anodes
-Current densities
Prevent Direct Anode/Cathode Contact
- Straight, rigid starter sheets or SS cathode blanks
- Insert starter sheets / blanks carefully
- Pull cathodes carefully
- If using starter sheets press 2 day cathode
- Remove warped anodes
- Anode / cathode insulators
-Buttons, hairpins, plastic runners, anode feet
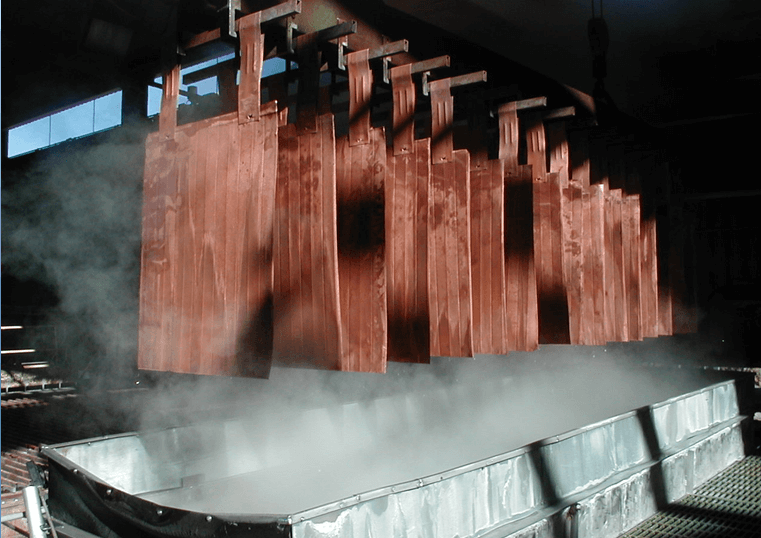
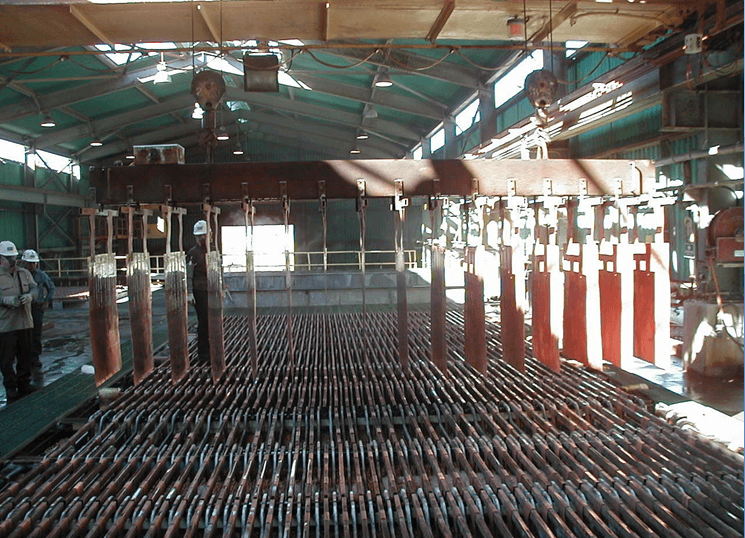

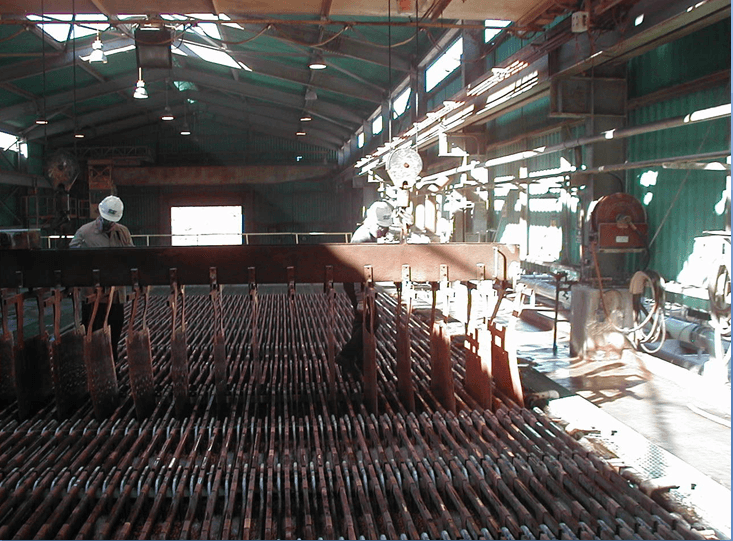
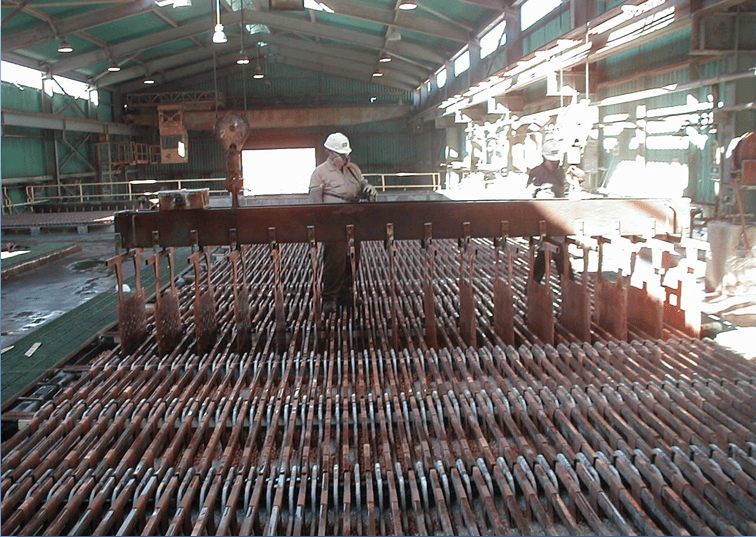


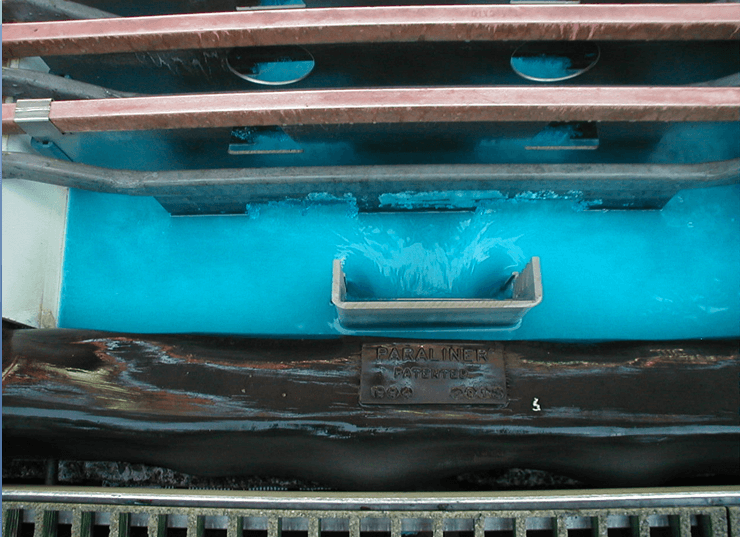
Wash Cathodes Effectively
- Immediate rinse when cathodes pulled
- Hot water soak (pull cycle)
- Cool down (pull cycle)
- Final hot water wash
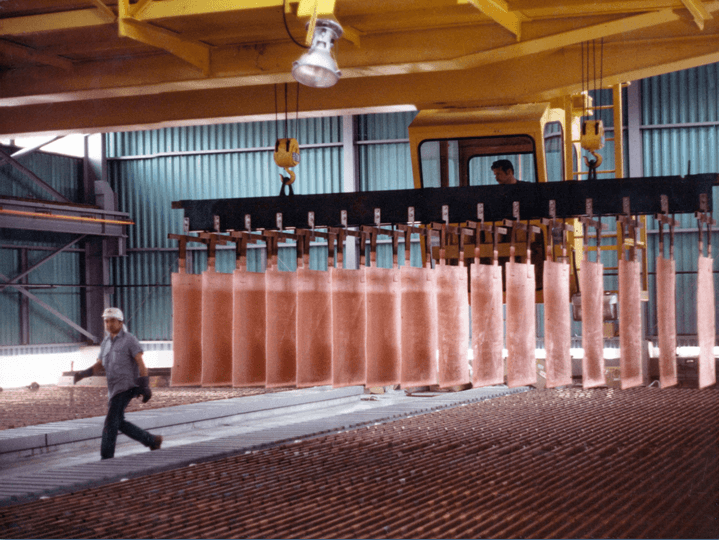
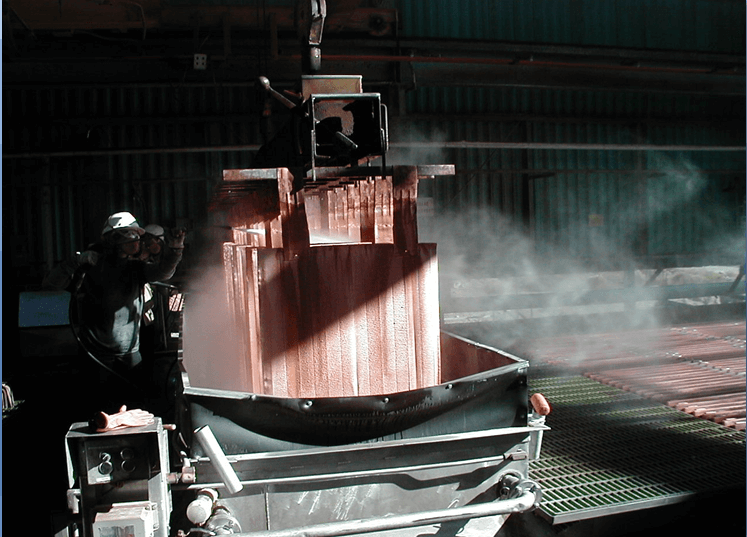
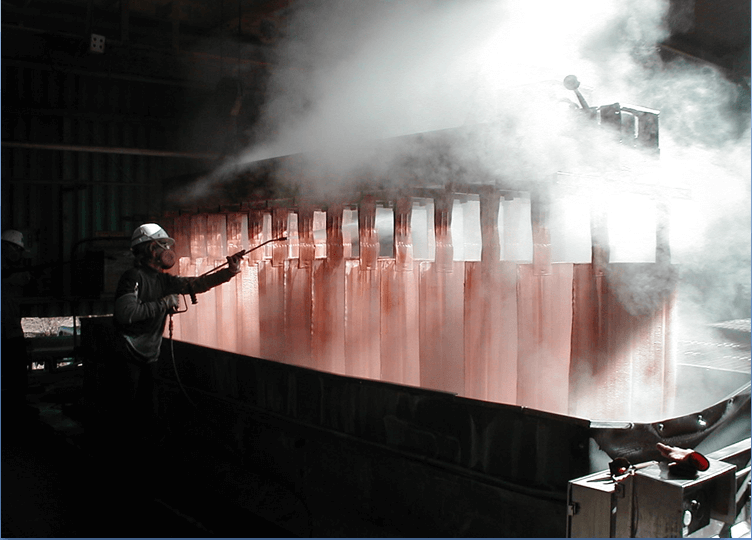

Other Considerations
- Run lower strip acid
-less corrosive acid mist
-longer anode life
-higher copper quality - Run higher copper pregnant electrolyte
-higher copper quality
-less entrainment of organic in electrolyte - Mist control – mechanical or chemical?












https://docslide.net/documents/copper-sx-training-nov-2007.html