Material of Vessels for Solution
The student must consider the effect of the solvent used on the vessel. In most cases the solvent used is an acid or mixture of acids, and for such solvents glass and porcelain are generally used. Platinum may be used, provided no chlorine or other attacking agent be present. (See notes regarding care and use of platinum crucibles.) For strong alkalies silver or nickel vessels should be used, as glass is attacked sensibly by hot and even cold solutions of caustic potash or soda, and porcelain is also attacked to some extent.
Methods of Solution & Apparatus used
When a substance is soluble in water and the solution does not require boiling, place the substance in a glass beaker, as at a in fig. 47. Add the water. Cover the beaker with a clock glass and heat. The solution may be boiled, but in this case it is better to perform the solution and boiling in an Erlenmeyer flask, as in fig. 47 at c.
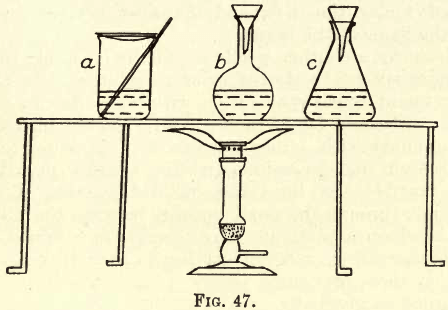
A funnel is placed in the mouth of the flask and through it the water is poured, whilst at the same time it serves for a cover. Or, again, the flask inclined will serve the same purpose. Where effervescence takes place on solution these methods serve to prevent mechanical loss through small globules of the liquid being projected over the edge.
If required, solution may be performed in a porcelain dish with an inverted funnel of smaller diameter than the dish, fig. 48, or a cover or a casserole (a deep porcelain dish with a lid and handle) may be used.
In most laboratories, as gas is available, heat can be applied by placing the vessel, after drying the outside, on a piece of wire gauze on an iron tripod about 8 inches high. The wire gauze may be replaced by a piece of thin asbestos board or a piece of wire gauze felted in the centre with abestos. The bunsen burner is then placed underneath and lighted, the colourless flame always being used. The Argand burner is shown in fig. 49.
Where much work is to be done, an iron plate (30 cm. x 25 cm. x 4 mm.) mounted on a four-legged stand is very convenient. If its surface is kept clean and glass vessels are wiped before transference to the hot plate, there is little danger of breakage. A few squares of thin asbestos board (about 10 cm. square) are useful for placing under a vessel to reduce the temperature. Sand baths are sometimes recommended, but the iron plate, with or without asbestos board or cloth, is a neater arrangement, and with a large bunsen (Fletcher Russell) gives a good range of temperatures. See fig. 47.
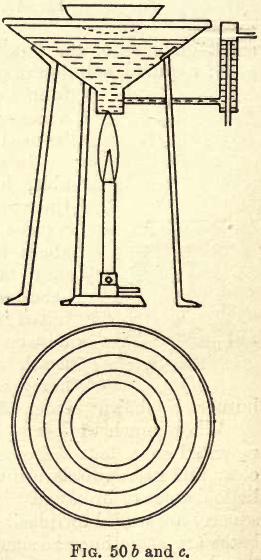
After solution, evaporation is frequently required. This may be carried out as shown in fig. 18, p. 7, the solution being transferred to the porcelain evaporating dish by pouring down a glass rod and washing the vessel and rod with a jet from the wash bottle. The beaker and porcelain, arranged as in fig. 50 a, are then placed on the hot plate, and the steam from the boiling water in the beaker heats the dish and slowly evaporates the solution.
In fig. 50 b and c are shown the elevation and plan of a self-filling copper water bath. Water is led in at the side and out at the bottom tube, and a constant level is maintained in the bath. The series of rings permits of considerable variation in the size of evaporating dish.

With care and practice evaporation may be carried out on the hot plate or tripod and gauze. The solution should never be allowed to actually boil; and towards the end of the evaporation, when the concentrated solution is liable to spit, a piece of asbestos board is placed under the dish, and the heat lowered by turning down the flame of the burner.
Certain substances are either partly or wholly insoluble in acids, and require special treatment prior to solution. As a rule, such treatment consists of fusion with some flux in a metal crucible. Silicates, for instance, are intimately mixed (when finely ground) with either carbonate of sodium or carbonates of sodium and potassium, and the mixture placed in a platinum crucible, the lid being inclined so that the contents are visible through the small opening between the lid and crucible. The crucible is placed on a platinum wire triangle on a tripod, and heat is applied by a bunsen burner till the charge just begins to melt round the edges. The foot blowpipe is then brought into position, the flame being turned on gradually till the whole mass is in a molten state. Care must be taken that the mass does not boil over. This may be prevented by carefully watching the contents through the opening and regulating the blast flame accordingly. This operation, unless otherwise directed, must be continued till the contents are in a state of tranquil fusion, and no blebs of solid or semi-solid matter are seen floating about. The flame is now turned out and the crucible and contents are allowed to cool. When cool, the crucible, contents, and lid are transferred to a beaker. Distilled water is then added and a few c.cs. of hydrochloric acid. The beaker and contents are then gently warmed till solution is complete, when the crucible and lid are raised up with a glass rod and washed with a jet of hot water from the wash bottle.
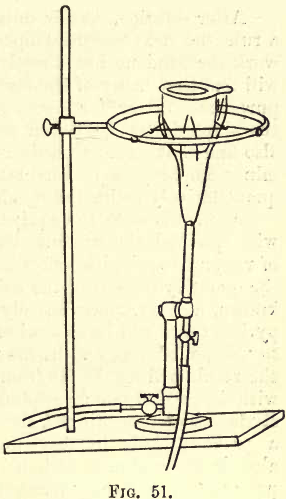
It will be seen in this case that by fusion the insoluble silica or silicate is changed into soluble silicates of sodium and potassium. As such a fusion takes from fifteen to thirty minutes with the foot blowpipe (see fig 51), this work will be found somewhat tedious, and where many fusions or ignitions with the blast have to be performed, it is advisable to replace the foot blower by a water blast. This piece of apparatus, in a very compact form, and suitable for both blast and suction, can be procured at a very moderate figure, and is easily attachable to the water service above a sink, the connection being made with lead or compo piping. This apparatus will serve for many purposes – ignition, fusion, filtration, glass-blowing, as an aspirator, etc.; and though rather expensive if provided for each student, one on every working bench can be recommended. Instructions regarding its manipulation are issued by the makers.
In fusion, as in solution, the effect of the flux (solvent) on the crucible must be considered. As a rule, porcelain crucibles are of little use, as the glaze is readily attacked, more especially by alkaline fluxes.
When using platinum crucibles certain precautions must be observed.
The following are some of the chief:
- Platinum is attacked by free chlorine, therefore it must never be used as a containing vessel for any mixture yielding free chlorine. Under certain conditions KClO3 will yield free chlorine. Similar remarks apply to bromine and iodine. Again, chlorides and nitrates in the presence of H2SO4 are liable to yield free chlorine.
- Compounds of silver, lead, tin, bismuth, antimony, and arsenic should under no circumstances be either ignited or fused in platinum vessels. Most of these metals have a low melting point, and when reduced from their compounds they readily alloy with platinum, and a probable result is that the bottom may be melted out of the crucible—that is, the destruction of a piece of apparatus worth from £3 to £6. In such cases porcelain crucibles should be used.
- Where caustic soda or potash or barium oxide is used as a flux, platinum vessels should never be used. To this might be added sodium peroxide, as, if impure, it frequently attacks platinum. In such cases silver or nickel vessels should be used.
- After a platinum vessel has been used for fusion or ignition, clean it by rubbing in the hollow of the hand with the fingers and a little sea sand the grains of which, under a lens, all appear rounded. For accurate work the surface must be kept in a bright state. Sharp sand must be avoided, as it scratches the surface of the vessel. Steins may be removed by fusion with KHSO4.
- When igniting or fusing, the vessel should always be supported on a triangle of platinum wire. This triangle may be made cheaply by running a thin platinum wire from the middle points of the sides of an iron triangle with sides about 7 to 10 cm. long. (Platinum foil wrapped round a pipe-clay triangle will answer.)
- A bad gas supply is liable to be the cause of a grey frosting on the outer surface of the vessel. Only the outer non luminous cone of the bunsen or blast flame should be used. Here the student is at the mercy of the gas company unless muffle furnaces are available. If a fusion be conducted in the muffle, any loose bone ash should be cleared away, and a small square of asbestos board inserted, on which the crucible is placed. When the fusion is complete, the board and crucible are gradually and carefully removed by the aid of a pair of cupel tongs.
- The student taking a course in metallurgical chemistry should possess his own crucible. Though expensive, it is always a valuable asset.
Saturated solutions
In the course of his work the student may require a saturated solution of some salt. Take a 200 c.c. Erlenmeyer or ordinary glass flask. Select a good cork, and through it fit two glass tubes, each bent at a right angle, one passing 3 cm. through the cork, and the other nearly to the bottom of the flask and slightly contracted at its lower end. Fill the flask about two-thirds full of distilled water. Add an excess of the coarsely powdered salt; insert the cork and fittings. Connect the shorter tube to an aspirator or the longer tube to the blast, and pass a steady current of air through the solution. If necessary, the solution may be aided by placing on a tripod and asbestos board and applying heat by a bunsen burner.
The same end may be obtained, but much more slowly, by ordinary methods, aided by shaking.
After solution, certain minor operations may or may not be necessary, but as a rule the next essential operation is that of precipitation.
When precipitation is complete the precipitate must be separated from the solution by filtration.
