Refining with Sulphur
A more speedy method of removing iron, copper, lead and base metals from bullion is to add sulphur to the molten metals. Although gold sulphides may be readily formed in the wet way, they are decomposed into gold and sulphur by merely heating them to a temperature below 300 deg. C. If pure gold is heated strongly with sulphur no combination of the elements appears to take place. Nearly all the other metals at a bright red heat unite readily with sulphur, and form sulphides. If a large proportion of copper be alloyed with gold then by granulating the alloy and heating gradually in a graphite pot a sulphide of copper or matte will readily form, and if the temperature is raised to melt the gold a button of gold containing up to 96 per cent., or even more, of the metal may be obtained. If attempts are made to convert the whole of the copper into a sulphide a large proportion of the gold will remain diffused through the copper matte, mainly in a finely divided condition, although part exists as a double sulphide of gold and copper. When silver is present as well as gold part of the silver passes into the matte as sulphide, but it is almost impossible to remove the whole of the silver from the gold button. When it is desired to add sulphur to an impure molten mixture of gold and base metals, the sulphur is sprinkled round the edge of the molten mass to prevent spitting, as would otherwise be the case if it were added at the centre.
The objection to adding sulphur in this way is that as soon as the sulphides form they float on the surface, so that any additional quantity added only volatilises unchanged. A better method was suggested by Mr. Manton, of Kalgoorlie. By melting the bullion first, and then having a smaller pot filled with sulphur, and inverting this into the molten mass, the sulphur vapour forces the metal as fast as it is formed from underneath the inverted pot to the space between the outer and inner pots. This method is open to the objection that the inverted pot chills and solidifies a crust of gold; the amount of sulphur which can be used with safety in this way is small, and there is danger of a violent volatilisation of the sulphur projecting the molten material out of the pot. By modifying the process in providing a means for a steady supply of sulphur into the inverted pot until the action is complete this method is one of the simplest and the most effective in cutting out most base metals from gold.
When the action has gone as far as desired it is better to allow the gold to solidify in the pot, most of the matte can then be poured off from the solid gold. If the matte also is allowed to solidify the crucible may be inverted, and the gold and matte dropped out. The matte, as a rule, can be knocked off. If the gold and matte are poured into a mould there is often the greatest difficulty in separating them. A complex matte, consisting as it often does of gold, silver, antimony, copper, and iron sulphides, clings persistently to the gold bar, and is not appreciably attacked by nitre or sulphuric acid. It is often noted that such impure bars sink very much in the centre on cooling; the matte runs in and fills up this longitudinal depression, making it still more difficult to get out. In order to remove this material probably the simplest way is to heat the bar to redness and sprinkle nitre on the matte until it ceases to glow. It is thereby loosened owing to the formation of sulphates, and may be more readily removed. By repeating the operation the gold bar may be rendered superficially clean.
While very impure bullion may be partly purified in this way very readily, yet it is not possible to remove appreciable amounts of silver and copper from ordinary alluvial or retorted bullion by this means. In other words, bullion containing only 10 per cent, of gold can readily be brought up to 90 per cent., but if the latter is taken, it is more difficult to raise it to 95 per cent, than to carry out the first separation.
The gold and silver present in the matte may be separated by adding some finely divided copper oxide, or even stirring round with an iron rod; portion of the copper sulphide is transformed to copper, which collects the gold and silver present into a button.
Refining with Sulphur and other Metals Together
The process of separating gold from silver and other metals by means of sulphur has been known for centuries, and has been fully described by Percy. It was commonly used up to the 18th century, and even now there are many important technical operations which the modern metallurgist should be acquainted with.
The first process described is by Theophilus, written in the eleventh century. The gold-silver alloy was granulated; a portion of the granulations was heated with sulphur at a low temperature. The sulphur formed sulphide of silver and melted. The gold for the most part remained intermixed, or even in solution in this molten mass. In order to precipitate it a further addition of the granulated alloy was added. This collected and precipitated the gold. The mass was heated strongly, allowed to cool in the pot; when cold the pot was inverted, and the solidified mass emptied out. The sulphide was separated from the metal by the blow of a chisel applied at the junction of metal and sulphide. The alloy, now very much enriched in gold, was cupelled with lead in order to separate the sulphides, and clean the metal. The resulting alloy was parted with nitric acid. The sulphides broken off were mixed with lead and cupelled. The silver, still containing a little gold, being thus recovered.
A modification of this process was to granulate the alloy; moisten it so as to cause the sulphur, which was mixed with the granules, to adhere more closely; to heat gently, and then more strongly, forming sulphide of silver. A small amount of iron was then added, or the mixture stirred with an iron rod, and the gold and portion of the silver was thereby collected. The separation of the gold and silver by means of litharge, cupellation, and nitric acid were the same as in the former example.
Another modification of these processes after the granulated alloy has been melted with sulphur, litharge is sprinkled so that the reduced silver will carry down the gold.
2PbO + 3Ag2 S – 2PbS + 6Ag + SO2.
Some lead is simultaneously reduced and alloyed with the gold and silver.
This operation has to be repeated until the silver sulphide is free from gold. The precipitated alloy after cupellation was treated with nitric acid. The regulus or mixed sulphides is melted with scrap iron to precipitate the silver and lead. The alloy was cupelled, and the iron sulphide sent to the blast furnace.
These methods, depending on the separation of gold from silver, were only applicable as concentrating processes. No attempt appears to have been made to obtain fine gold by this method of treatment. From experiments made by the author on granulated alloys, also very fine alluvial gold, having a high ratio of gold-silver; very little silver is removed from such alloys by means of sulphur alone. The gold present in the sulphides, such as sulphide of silver, sulphide of copper, sulphide of iron, appears to be in the state of a sulphide also, or else it is in solution, while these are molten, and remains, disseminated when the sulphides are cooled.
On heating about 50 oz. of auriferous copper scalings with sulphur, and melting at a high temperature, only a small proportion of the gold separated in the form of a button. On re-melting with more copper scale practically the whole of the gold was precipitated, and this gold contained very little copper. This would indicate that the gold was in the form of a sulphide, and was displaced by the copper. On the other hand, when the rich auriferous regulus was broken, bright scales like gold could be seen. This apparent separation of gold may, however, have taken place on cooling in the same manner as metallic copper will separate in moss-like form from copper matte on cooling; similarly metallic silver will separate in spongy, or hair-like forms from sulphide of silver formed as indicated in the preceding cases, when the mass becomes cold.
On dissolving such mattes in nitric acid, part of the gold sometimes appears as if it were unaltered, and had not combined with the associated elements, but on the other hand B sometimes the whole of it is obtained as a brown, spongy mass such as is left when gold compounds or gold alloys are acted upon by acids such as nitric. It would therefore appear that although gold sulphide is readily decomposed at a moderate temperature into gold and sulphur, that when it is intermixed with other sulphides containing excess of sulphur that it dissolves in them, and that, on cooling, part of it is rejected as metallic gold, but part remains either as metal in a solidified solution or as a sulphide.
Refining with Sulphur and Sodium
From experimental work done, the author was driven to the conclusion that a carrier is needed for the sulphur and silver alloyed with gold. The effect of sulphur itself is transient when its vapour comes in contact with molten gold containing silver. If it were forced through the molten alloy its effect would be more marked, but it is doubtful if it will quantitatively remove the silver from the gold. Since its action is more effective when metals having a great affinity for it are present, an experiment was tried with metallic sodium and gold, to which sulphur was added. The alloy of sodium, silver and gold is very liquid at a low temperature. The formation of the alloy was first attempted by melting the gold with sodium in a cavity scooped out of a charcoal block, but the alloy formed was so fluid as to soak into the pores of the charcoal. Alloys were subsequently made in hard glass tubes, or in porcelain crucibles enclosed in plumbago ones. By heating gradually, with exclusion of air, the gold rapidly dissolved in a comparatively small weight of sodium, and formed a uniform alloy. On adding sulphur to this alloy the sulphur united with the metals with great vigour, raising them to a very high temperature.
The first qualitative experiments showed that silver could be removed by this method as well as base metals, and quantitative tests were made to see what actually took place when the alloy was subjected to this treatment. An alloy consisting of
72.14 gold
27.82 silver
0.04 copper
was taken and mixed with sodium in the proportion of 10 to 1, or 10 grammes of the alloy and one gramme sodium.
The mixture was placed in a porcelain crucible; which was put in a clay crucible, a large porcelain lid of a crucible was inserted into the clay pot, leaving ample room between it and the top of the porcelain crucible. When the pot became red hot small pieces of sulphur were dropped on the porcelain lid. They rapidly melted and trickled down into the lowest compartment, thereby coming in contact with the molten alloy. When the pot had been raised to a full red heat it was withdrawn from the fire and allowed to cool. The porcelain crucible was lifted out, and placed in water and boiled. At first a strong yellow solution formed, with a few black specks through it. This was filtered, and the filtrate kept. Fresh water was added, and the metal and matte boiled until nothing more dissolved. The fused mass became detached from the crucible, and consisted of a button of gold below and a dark, waxy-looking layer above. The upper surface of the latter had a film of gold, no doubt due to some air getting access to the crucible on cooling. The gold button was detached and weighed. Its weight was 3.25 grms. It consisted of gold and silver in the following proportions:
gold, 87.1
silver, 12.9.
The standard had, therefore, been raised about 15 per cent., but instead of having 7.21 grms. of fine gold there was only 2.83 grms., the balance having passed into the matte. Again it would be thought that the gold from this would have been precipitated by the free silver still present in the button. This may possibly be the case if the mixture is kept molten for a considerable time, for previous experiments indicated that a higher grade gold can be obtained by this means, and a smaller proportion passed into the matte.
To the solution from the matte dilute sulphuric acid was added until it was acid. At first a port wine colour formed, but on adding more acid, a brown precipitate fell, and sulphuretted hydrogen was evolved, after a little time a white precipitate of sulphur came down—the precipitate was filtered, dried and heated, and was found to consist of sulphide of gold. The weight of gold obtained from this was 0.431grms., this was 992 fine. The weight of pure gold was, therefore, 0.4275grms. The remainder was silver.
The sulphide matte remaining after washing weighed 6.97 grammes. On being broken the matte had a columnar fracture. It was examined with a glass, but no gold could be seen in it save the film before mentioned. The matte was gently washed in a porcelain crucible. Strong sulphuric acid, and subsequently bisulphate of soda, were added, heated, washed, and the gold and silver collected separately.
After the fusion with bisulphate the gold hung together after the manner of retorted gold; it had the same apparently uniform external crust, and the same sponginess inside, and the same tenacity. In fact it was undistinguishable from it. After washing the silver out, the residue was smelted in a clay pot with phosphoric acid and a small quantity of bisulphate of soda. The resulting gold assayed 996, and weighed 3.92 grammes. The slag resulting from this fusion was a magnificent ruby color by reflected light, and some of it this color by transmitted light, and some a rich violet blue, resembling very much solutions from which gold has been precipitated. It contains a small quantity of gold, which probably gave the color to it. A summary of the forms in which the gold existed is as follows:—
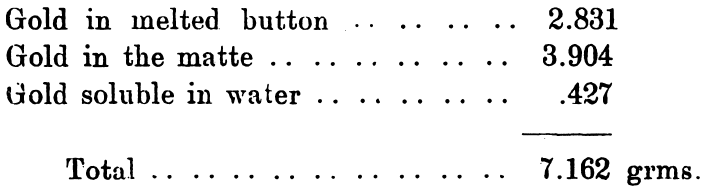
The amount originally present was 7.21grms., so that 0.05 grms. are not accounted for here. On testing the silver obtained from the matte it was found to contain a minute proportion of gold, there was also gold left in the slag, and when sulphur was added to the gold sodium alloy sparks were evolved, so that some would thus escape treatment.
Sulphide of gold, soluble in water containing sodium sulphide, always appears to carry a little silver through with it, since the gold obtained after cupellation is not fine.
In view of the readiness with which sulphur, in conjunction with sodium, would attack gold and silver, it seems a roundabout way of making the material, when sulphide of sodium with excess of sulphur might be added itself to the molten alloys, yet experiments made on these lines gave no results at all like the previous one. The gold and the silver remained almost unattacked when melted with sulphide of sodium to which sulphur was from time to time added. Several more crucial experiments were made by taking cyanide slime precipitates containing 14 per cent, of gold in a fine state of division. These were heated with sulphide of sodium, anhydrous thiosulphate of sodium, and poly sulphide of sodium. In each case five grammes of slimes was taken, and 5, 10, 15 grammes of the sulphur compound. They were heated in a closed crucible to redness, and then washed with water. The solution was acidified, a precipitate of sulphur formed H2S was given off. The precipitate was dried carefully, burned, and wrapped in sheet lead and cupelled. In no case was there more than a trace of gold in solution, and accompanying it was as much silver. The proportion of gold dissolved could be accounted for by the action of thiosulphate of sodium which forms when the solution of alkaline sulphides are brought into contact with the air. These results surprised me, since it has generally been accepted that gold is readily soluble in fused alkaline sulphides. Time did not permit of following out this line of investigation, but the subject will be dealt with more fully later on.
As far as researches have gone at present it seems necessary to have an alloy with the gold which has a great affinity for sulphur, in order to remove the silver in the state of sulphide. The alloying metal acts as a carrier of sulphur throughout the mass of molten metal. It may be that other investigators found that the alkaline sulphides attacked gold by actually reducing some sodium compound to metallic sodium in contact with gold; this would alloy with the gold, and if sulphur was present at the same time the effect would be the same as described on adding sulphur to a prepared alloy.
Refining using Sulphide of Antimony
This process is also some centuries old, and is based on the fact that when gold-silver alloys are melted with sulphide of antimony sulphide of silver will form, and the gold will alloy with the antimony.
Au + 6Ag + Sb2S3 = Au + 2Sb + 3Ag 2S
Copper and other metals will also be removed by this process. It differs from the method of refining by sulphur alone in that high grade bullion can be taken and refined to more than 99.3 per cent gold. It is stated:—“It is sulphur that is really the agent by which silver is separated from gold in this process; but, as it is in combination with antimony, it may be kept in contact with the alloy of those metals at a much higher temperature than is practically possible when it is uncombined, the presence of antimony not interfering, because it has a less affinity than silver for sulphur.” Since the heat formation of antimony sulphide is 34 (Sb2 S3), and of silver sulphide (Ag2 S) is 0.3 or 3 Ag2 S = 9, the explanation given is not complete. The affinity of gold for antimony, and the high temperature must also be taken into account. The influence of mass has also to be considered, for it is not possible to carry on this operation with the equivalent of sulphur necessary for the silver.
The quantity of sulphide of antimony to be added depends on the amount of silver present, and the amount of base metal, such as copper. This amount varies from twice in the case of high grade bullion, to four times in the case of baser bullion. The alloy is first melted, and then sulphide of antimony is added. The crucible should not be more than two- thirds full on account of the danger of frothing over. As soon as sparks are emitted, the mass is poured, and the sulphide detached from the gold antimony alloy, which still contains silver. The latter is again melted with an additional quantity of stibnite equal to twice the weight of the alloy: finally the operation is repeated with an equal weight of antimony sulphide. The operation is repeated if necessary until the gold is of the desired standard. Some of the gold remains in the fused sulphide. This is separated by fusing the latter for a considerable time, when the gold alloyed with silver separates. This button is treated in the same manner as the bullion. The antimony is separated from the gold by blowing a current of air over the surface of the molten metal. As the antimony is removed the melting point rises, and at the finish nitre and borax are added to remove the oxides and give a clear surface to the molten gold. The sulphides are treated by melting them with lead and iron. The resulting lead is cupelled, and the silver obtained is parted with nitric acid; the sulphide of antimony and iron have to be further treated to extract the silver still contained. This process was in use up to 1846, but is too costly as compared with the sulphuric process, or the Miller process of refining high grade bullion. There are also too many operations in the process, too many by¬products, and the gold is not wholly separated from the silver in the final matte, nor is the silver from the gold in the final gold product.
Not only will sulphur serve for the separation of silver from gold when much silver is present, but other sulphides with the addition of sulphur also serve the same purpose. Sulphide of copper, for example, with sulphur will remove a considerable part of silver from gold, but the operation must be repeated several times in order to obtain gold of high standard. In this case also the gold-silver should be melted with copper, if it is not present already, the alloy granulated and the granules moistened, mixed with sulphur as before, and the whole mixture heated gradually but finally to a high temperature. The matte is broken off and the operation repeated on the alloy, with, if necessary, the addition of more copper. A considerable amount of the gold also passes into the matte; this may be precipitated along with the silver by the addition of some iron.
There is no doubt that this method is the simplest for the treatment of the cupriferous bullion often obtained in cyanide precipitates.
