This process has now, in the majority of refineries, superseded the nitric acid method, which is much more expensive, owing to the higher cost of the acid used and of the plant required. The German chemist, Kunckel, who lived in the seventeenth century, is said to have been the first to employ sulphuric acid in parting, but it was not used on the large scale, when it was introduced into France, and worked in a refinery built in Paris for the purpose. It was established in London at the Mint Refinery by Mr. Mathison, and has been in almost continuous use there ever since, with little change, the refinery having been let on lease by the Government.
The method used varies considerably in different refineries, but essentially consists of the following operations:
- Mixing and granulating the alloys.
- Dissolving the silver from the granulations by sulphuric acid.
- Washing and melting the gold residue.
- Precipitating the silver from its solution by means of copper.
- Recovering the copper sulphate by crystallisation.
The account given below is a general view of the operations in various refineries, the modifications adopted not being described in most cases.
Gold Mixing and Granulating
The alloys must be carefully prepared so as to be of suitable composition, as otherwise difficulties are encountered. The most suitable proportion of gold in the alloy is said by Dr. Percy to be from 18 to 25 per cent., including whatever copper there may be present; but some American writers consider the proportion of one part of gold to two and a-half parts of silver to be the most desirable, whilst at a refinery at San Francisco the alloy consists of two parts of gold to three parts of silver. This proportion was instituted when alloying silver was scarce in California and has never been abandoned, but the gold thus separated is only 990 fine, containing ten parts of silver, the maximum allowed by law in the gold coins of the United States. If the ordinary proportion of three parts of silver to one of gold is used, however, the gold can be obtained about 996 fine, and the fineness of the gold can always be increased to about 998 or 999 by fusing it, first with bisulphate of potash and subsequently with nitre. If there are only a few parts of gold per 1,000 of the alloy, it has been stated that the silver left in the gold amounts to as much as 3 or 4 per cent.; nevertheless, such an alloy, when subjected in the form of bars to the action of the acid, instead of being granulated, yields gold at San Francisco of no less than 996 fine, after one boiling only.
The amount of base metals present in the alloy must be carefully regulated, as their sulphates are little soluble in concentrated sulphuric acid, and consequently are precipitated and interfere with the progress of the operation. Bars consisting in great part of copper are often received at the San Francisco works. These are melted with fine bars so as to reduce the proportion of copper, which must not be more than about 10 or 12 per cent, of the whole; a small amount of copper facilitates the solution of the silver. A small quantity of lead is said to assist in the solution of the copper, which is somewhat slowly attacked by concentrated sulphuric acid, and a maximum amount of 5 per cent, of lead does not interfere with the operation. From the economy with which this system of parting can be practised, silver containing only 0.5 part of gold per 1,000 can be separated from it at a profit, while the nitric acid process is unremunerative if applied to an auriferous silver alloy containing one part of gold in a thousand. At the Vienna Mint, bars are parted containing 0.9 part of gold per 1,000, and at Freiberg bars containing only 0.4 part per 1,000 are profitably treated.
In England, silver bars are passed through the parting operation, if they contain at least 2 grains of gold per troy pound, or 0.35 part per 1,000, but dore silver is not parted by itself. It is mixed with rich gold alloys.
The parting alloy is usually granulated, but at the San Francisco Refinery the dore silver is not granulated but melted and cast into bars ¾ inch thick, 9 inches wide, and 15 inches long.
Solution of the Silver
This is usually effected in cast-iron kettles, platinum having been abandoned on account of its high cost. The iron used is fine-grained compact white iron, preferably containing 3 or 4 per cent, of phosphorus, which increases the durability, although 2 per cent, only of phosphorus is considered enough by some refiners. 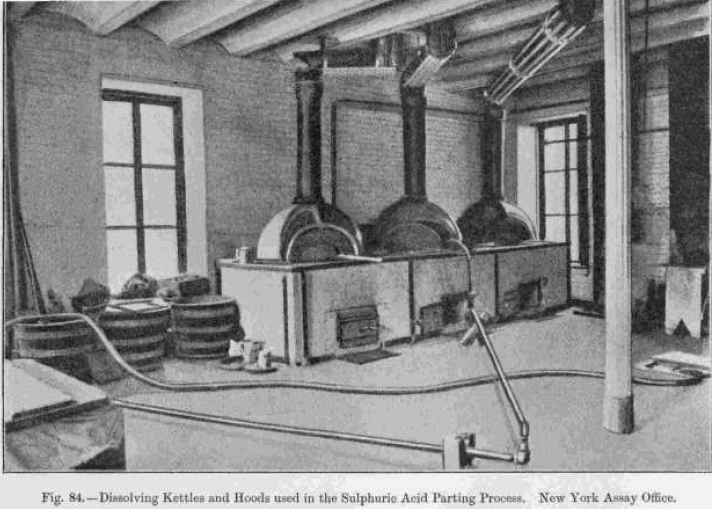 The kettle is slowly dissolved by the acid, ferrous sulphate being formed, and, in the course of about two years, the thickness of the vessel is reduced from about 2 inches to from ¼ to ½ inch, when it is discarded. The perfect exclusion of air from the interior increases the length of life, and dilute acid must not be allowed to come in contact with the iron, as the latter is freely dissolved by it. The vessels are rectangular or cylindrical, with flat or hemispherical bottoms, the latter being preferred in Europe and the former in America. They are covered with cast-iron lids, about ½ inch in thickness, which are bolted tightly to the vessels, and have bent leaden pipes fitted to them for carrying off the fumes, which consist largely of SO2. This is sometimes reconverted into sulphuric acid in leaden chambers arranged for the purpose. The cover has also an opening (supplied with a lid made air-tight by a water joint) through which the alloys and acids are added and the operation watched. Heat is usually supplied by a wood fire (see Fig. 84).
The kettle is slowly dissolved by the acid, ferrous sulphate being formed, and, in the course of about two years, the thickness of the vessel is reduced from about 2 inches to from ¼ to ½ inch, when it is discarded. The perfect exclusion of air from the interior increases the length of life, and dilute acid must not be allowed to come in contact with the iron, as the latter is freely dissolved by it. The vessels are rectangular or cylindrical, with flat or hemispherical bottoms, the latter being preferred in Europe and the former in America. They are covered with cast-iron lids, about ½ inch in thickness, which are bolted tightly to the vessels, and have bent leaden pipes fitted to them for carrying off the fumes, which consist largely of SO2. This is sometimes reconverted into sulphuric acid in leaden chambers arranged for the purpose. The cover has also an opening (supplied with a lid made air-tight by a water joint) through which the alloys and acids are added and the operation watched. Heat is usually supplied by a wood fire (see Fig. 84).
The charge for the pots varies from 200 to 1,000 lbs. of alloy, and the amount of acid required varies from 2 to 2½ times the weight of the alloy, depending on the composition of the latter. About one-half of the acid, which is strong commercial acid of 66° Beaume (sp. gr. 1.85) is added at first, and the temperature cautiously raised to boiling point, when the pot is closely watched, and, if the ebullition becomes too violent, the temperature is lowered by regulating the fire and by adding cold acid a little at a time. The charge is stirred occasionally with an iron tool, particularly towards the end of the operation, when the undissolved granules of metal must be freed from the surrounding sediment, consisting of sulphates of the base metals, and exposed to the action of the acid. The ebullition gradually subsides and action ceases in about five or six hours, the presence of a greater proportion of base metals increasing the length of time required. The reactions are as follows:
- 2H2SO4 + Ag2 = Ag2SO4 + SO2 + 2H2O
- 2H2SO4 + Cu = CuSO4 + SO2 + 2H2O
and similar reactions with tin and lead. The reactions with antimony, bismuth, zinc, and iron are more complicated. It is obvious that 63 parts of copper decompose as much sulphuric acid as 216 parts of silver. It is clear, therefore, that an increase in the percentage of copper present necessitates an increase in the amount of sulphuric acid required.
One part of sulphate of silver is soluble in ¼ part of boiling concentrated sulphuric acid, but the solubility rapidly falls off as the temperature and concentration diminish, so that 180 parts of cold acid of specific gravity 1.08 are required for the same purpose. Sulphate of copper dissolves slightly in the boiling concentrated acid, but is almost all precipitated in the form of the white anhydrous salt on cooling. Tin and zinc behave similarly, and lead makes the solution turbid and milky. The iron would not be so much attacked if it were not for the increasing dilution of the acid during the process, owing to the formation of water, which is, however, in great part boiled off as fast as it forms, or taken up by the anhydrous sulphates. The presence of copper checks the dissolution of the iron.
When the dissolution is complete, the charge is ladled into a settling pot and a few pounds of cold acid of 55° B. are added, by which the acid is cooled and diluted, and some crystals of silver sulphate are formed. These, falling to the bottom, carry down with them the suspended fine particles of gold, and so clarify the solution. If much copper is present, however, this is not necessary, as the slight cooling of the acid is enough to precipitate some sulphate of copper, which falls to the bottom and adheres to it very firmly, thus clarifying the liquid and enabling it to be poured off or ladled out very closely. The clear silver solution is then ladled out with iron ladles into lead-lined rectangular wooden vats already partly filled with hot water, in which the precipitation is subsequently effected.
Washing and Melting the Gold Residue
The residue in the dissolving pot, if the amount of base metals present is not large, is then boiled twice more with fresh concentrated sulphuric acid added hot, after which the gold residue is hard and heavy and rapidly subsides to the bottom, and the liquors are ladled into the precipitating vat. The gold is dipped out with an iron- strainer and transferred to a lead-lined filter-box where it is thoroughly washed, first with hot dilute sulphuric acid and subsequently with boiling water, after which it is pressed, dried, and melted. It is almost always brittle, from the occurrence in it of traces of lead or tin, which are difficult to separate by sulphuric acid owing to their insolubility. These metals are eliminated by fusion with nitre or by a blast of air, and the bars thus toughened.
If the amount of base metals present is very large, the gold residues are ladled into a vessel of hot dilute sulphuric acid and boiled with it by means of steam. In this way, most of the sulphate of silver and the whole of the copper, zinc, iron, &c., remaining with the gold are rapidly dissolved. Care must be taken, however, to add the residues a little at a time, as otherwise the anhydrous sulphate of copper will form lumps, which are only slowly dissolved. The gold is then allowed to settle, and, after the solution has been drawn off, is boiled again with acid if necessary, or if it is already pure enough it is at once washed, dried, and melted.
In Europe it is not customary to attempt to obtain pure gold from auriferous silver in one operation, but the gold is concentrated in a small quantity of silver and then mixed with other alloys rich in gold and parted again. The product of gold thus obtained is purified by heating in a furnace in small iron pots with about half its weight of bisulphate of potash, by which some additional silver is converted into sulphate. The temperature is not raised much above the fusion point of the salt. The fused mass is then boiled in sulphuric acid, and again washed, dried, and melted. In the United States these methods are not used, auriferous silver being cast into slabs and parted merely by boiling with sulphuric acid; fusion with bisulphate of potash is rarely resorted to, but at the United States Mints, the residues are boiled with fresh acid five times in succession.
Precipitation of the Silver
On pouring the sulphuric acid solution into water, most of the silver sulphate is precipitated at once in the form of small crystals, consisting of bisulphate, and the liquid must then be raised to boiling, by means of steam, in order to redissolve them. When the original alloys contain much lead this is not redissolved, and it is, therefore, necessary to let the solution settle and transfer the clear liquid to another vessel. Some particles of gold are usually found in the precipitate thus formed.
The reduction and precipitation of the silver are effected by means of copper, which takes its place in solution. The copper is usually added in the form of scrap while the liquid is being heated up by steam. The precipitation is assisted by constant stirring by means of wooden paddles. In San Francisco, however, the copper is cast into slabs, which are suspended side by side in the solution in a vertical position. The solution should be of about 24° B.; if it is much more concentrated than this, the precipitation of the silver is imperfect. The end of the reaction is detected by testing with salt solution, and when complete the stirring is stopped, the solution allowed to settle for two hours, and the clear liquid tapped into lead-lined vessels, where further settling of the suspended particles of silver takes place. The precipitate of silver is thoroughly washed with boiling water in wooden filters lined with lead, until the reaction for copper can no longer be obtained with ammonia. Care must be taken that no fragments of metallic copper remain with the silver. The metal is then pressed, dried, and melted, and is usually from 998 to 999 fine, even without fusion with nitre, when the copper plates are used for reduction.
Crystallisation of the Sulphate of Copper
This is effected by alternate evaporation and crystallisation in lead-lined wooden tanks. The solution, which is still of 24° B., is run from the precipitating tank into the evaporating pan and concentrated to 40° B. by heating with steam; thence it is transferred to the crystallising tanks, where it is allowed to cool and remain for from ten to twelve days. The mother liquid of 36° B. is then run off and concentrated to 45° B., after which it is again allowed to crystallise, reconcentrated to 55° B., and a third crop of crystals obtained, which contain much iron. The clear acid mother liquor can now be used to dilute the solution of sulphate of silver in the dissolving pot as already described. The excess of acid in surplus liquids is neutralised with oxide of copper, more copper sulphate being thus formed.
The crystals of bluestone are found adhering to the sides and bottom of the tanks. They are detached with copper chisels, redissolved in pure water and recrystallised, the mother liquors being eventually added to the first liquor from the precipitating vats. When the liquors become over-charged with iron, the copper in them is precipitated by means of metallic iron, and they are thrown away or evaporated to get the crystals of sulphate of iron. The bluestone crystals are packed in barrels for the market. One pound of metallic copper with 1.5 lbs. of sulphuric acid of 66° B. will make 4.5 lbs. of crystallised sulphate of copper.
The cost of the process of parting by sulphuric acid in Europe is about one farthing per ounce troy of the parting alloy.
