 The Tavener process of melting the gold precipitate in a reverberatory furnace with litharge, thus forming a lead bullion which was afterward cupelled in the usual way. He deals with the zinc shorts by the same method but recommends that the two products be partially dried and separately fluxed. For the precipitate he uses the following mixtures:
The Tavener process of melting the gold precipitate in a reverberatory furnace with litharge, thus forming a lead bullion which was afterward cupelled in the usual way. He deals with the zinc shorts by the same method but recommends that the two products be partially dried and separately fluxed. For the precipitate he uses the following mixtures:
Product……………………………………100 parts by weight
PbO………………………………………….40 to 60 parts by weight
Assay slag………………………………….10 to 15 parts by weight
Slag from previous melts……………10 to 15 parts by weight
Sawdust……………………………………½ to 1½ parts by weight
These ingredients are mixed and rubbed through a sieve and then shovelled into the reverberatory before starting the fire. Regarding the amount of litharge used, Tavener recommends that it should be adjusted to produce a lead bullion containing not over 8 to 10 per cent. of precious metal. The short zinc is fluxed differently.
Zinc…………………………………………….100 parts
PbO…………………………………………….100 to 150 parts
Mixed slag……………………………………25 to 30 parts
No sawdust is added here. This mixture is spread over the charge already in the furnace, and on that a sprinkling of litharge and overall a thin layer of easily fusible slag. The fire is then lighted and the temperature gradually increased and maintained at a high point until fusion is complete. The charge is then stirred well and sawdust added to reduce any litharge that may still remain in the slag. The liquid slag is tapped or rabbled off to within an inch of the metal surface and the remainder thickened and carefully skimmed. The clean lead is tapped off into bar moulds of a suitable size for feeding into the cupel furnace, which is of the usual type.
In the case of silver it is usual, after cupellation is finished and the molten surface bright and clean, to ladle the bullion out into moulds but in dealing with gold where a depth of only ½ to one inch of metal remains in the cupel after the operation is complete, it may be cooled just to the point of solidification; then an iron bar gently placed under the edge of the sheet of metal and pried upward will cause it to break into two or more pieces which may be removed with tongs: if these pieces be at once tapped with a hammer before cooling further they will break up into small pieces of any desired size for charging the crucible in which the shipping bars are produced.
At the Homestake the precipitate after acid treatment is mixed with litharge, borax, and assay slag, and briquetted. The briquettes are dried in a furnace to a point where they can be charged on a bath of molten lead without spitting. In this case the melting and cupellation are done in the same furnace, which is an English-type cupel furnace with oval test, made of a mixture of cement and limestone in the proportion of 75% to 25%. After the fire is started lead is fed into the furnace and when a bath of well-heated metal has been obtained the briquettes are fed in on top, more being added as they melt down, until the test is full of fused material. The slag is tapped off by cutting down the front wall of the test, the aperture closed, and more briquettes charged in, the operation being repeated until all the precipitate has been disposed of. Finally more lead is added to raise the level of the bath and the slag and matte remaining finally cleaned off. Cupellation is then proceeded with until all the lead has been eliminated and only fine bullion remains.
The Tavener process is a large-scale version of the well-known fire assay procedure, which uses lead to collect and separate precious metals from a molten charge. The precious metals are then recovered by vaporization (cupellation) of the lead or less commonly by dissolution of the lead in acid. The process was used widely until about 1950, and is still used occasionally for the treatment of low-grade materials and materials with high lead content, for example, lead cathodes from some electrowinning processes.
Fluxes, lead oxide (PbO, or litharge), and. if necessary, carbon are mixed with the charge of material to be smelted. Smelting is performed at similar temperatures to those required for simple flux smelting. The lead oxide is reduced by metals, sulfides, and carbon, if present, to form lead metal. The lead drains through the molten charge as fine droplets and collects the contained gold and silver. The collection process is highly efficient provided that the ratio of lead-precious metals is kept high, typically >10:1. The lead-precious metal alloy is tapped out of the crucible and cooled. This alloy is then treated by cupellation in a strong stream of air to oxidize the lead back to lead oxide, which is removed as a vapor and can be recovered as a solid on cooling to allow recycling to the fusion stage. The gold and silver remain, and can be poured or remelted into bars.
The process is slow, primarily because it requires two stages; it is labor intensive; and lead fumes are produced, which present a health hazard. Despite these disadvantages, the process is effective for treatment of some low-grade refinery products.
Litharge Recipe
In this process the zinc-box precipitate is filter-pressed, the fine zinc having previously been separated. The slimes are partly dried, rubbed through a coarse sieve, mixed with fluxes, again sieved, and shovelled into a reverberatory furnace. The fine zinc is also mixed with fluxes, and charged in on the top of the slimes. A cover of litharge and old slag is then added, and the charge is ready for smelting. The proportions were as follows:
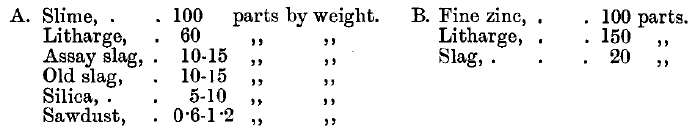
The fire is then lighted, and the charge slowly dried and melted. Very little zinc is volatilised. The slag is stirred and more sawdust added, and when the slag is judged to be free from litharge, it is run off into slag-pots. The last of the slag is removed by skimming. The lead is then stirred, sampled, and drawn off into moulds. The usual charge yields about 12,000 ozs. of soft lead, containing about 8 per cent, of gold.
In the second operation the lead is cupelled in an English cupellation furnace. It would probably be better to make two operations of this, raising the fineness to 50 or 60 per cent, of gold and silver in one furnace and finishing in another, but Tavener does not propose this. The result is that the rich litharge flowing off at the end of cupellation is not kept separate. It is also stated that it is difficult to prevent the charge from freezing when most of the lead has been removed. The cupelled gold is allowed to set on the test, and is then prised off, broken up while hot, and melted in crucibles. Tavener states that the gold and silver carried off by the litharge is not lost, as the litharge is used over again. There is a limit to this system, as the copper would not be slagged off in the reverberatory furnace and would accumulate in the litharge, which would eventually become too impure for further use.
The losses in the process are unknown, but are believed to be far less than in the methods previously described. The cost of treatment is stated by Tavener to be about 3d. per oz. of gold in South Africa, as against about 1s. per oz. for the acid treatment, calcining, and smelting process.
