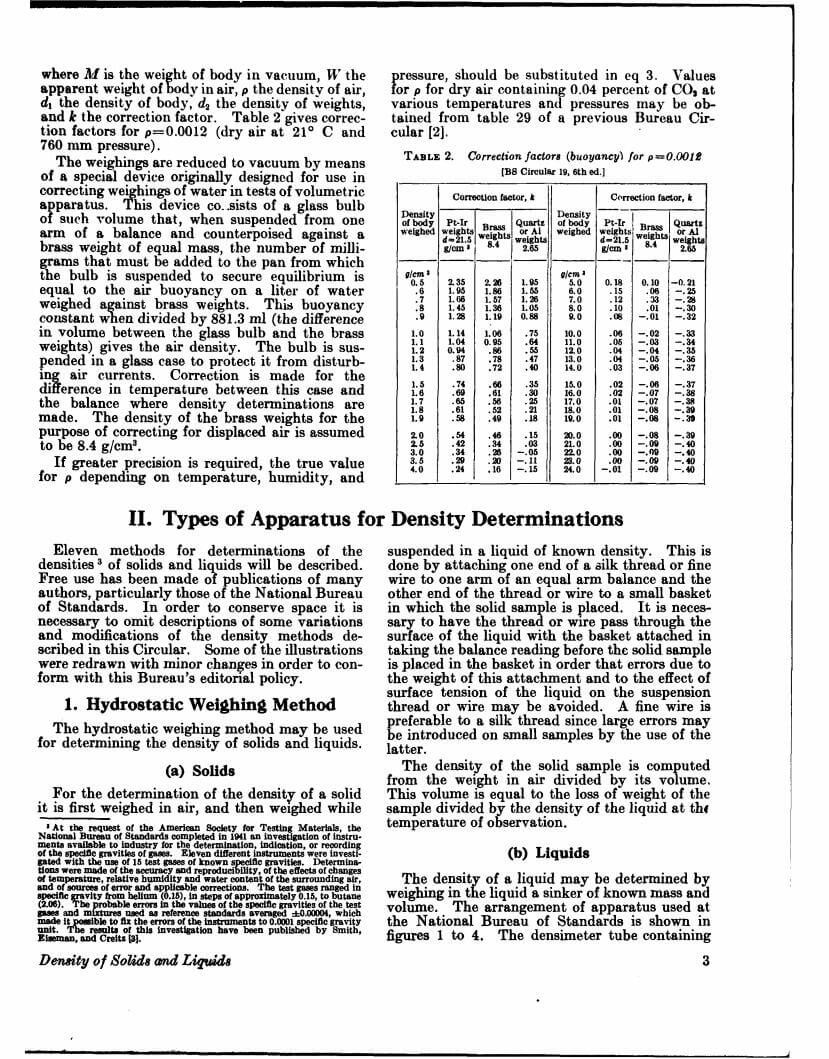
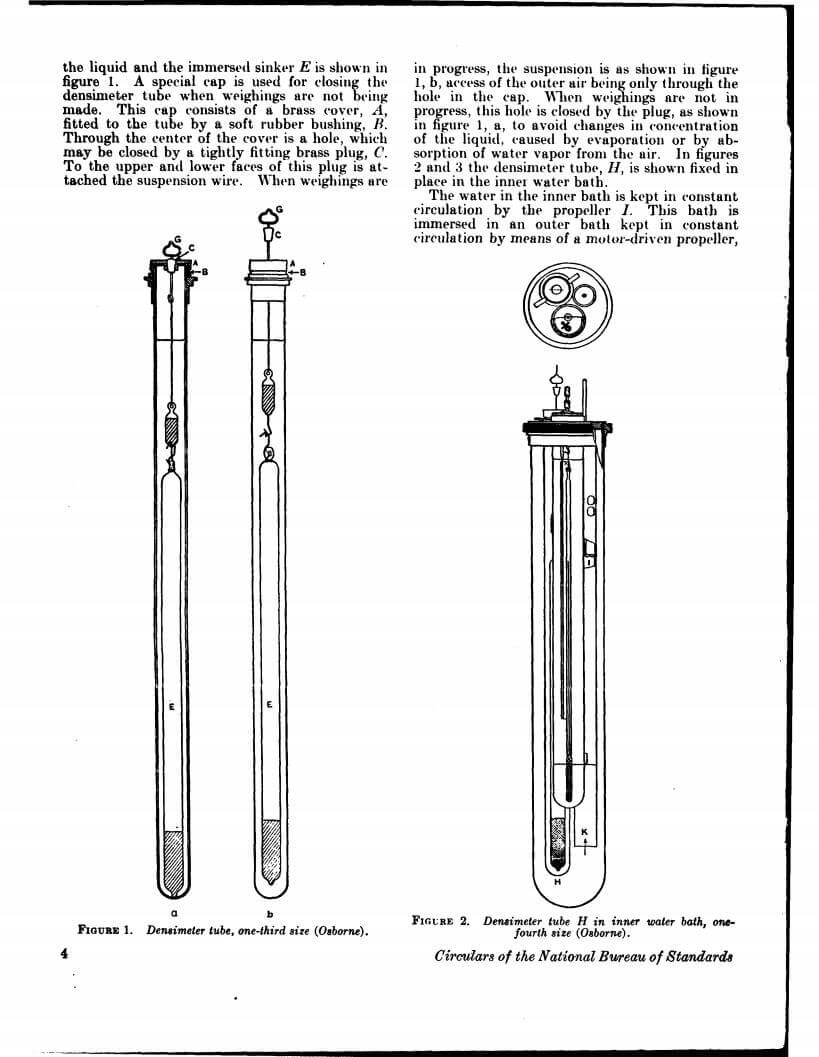
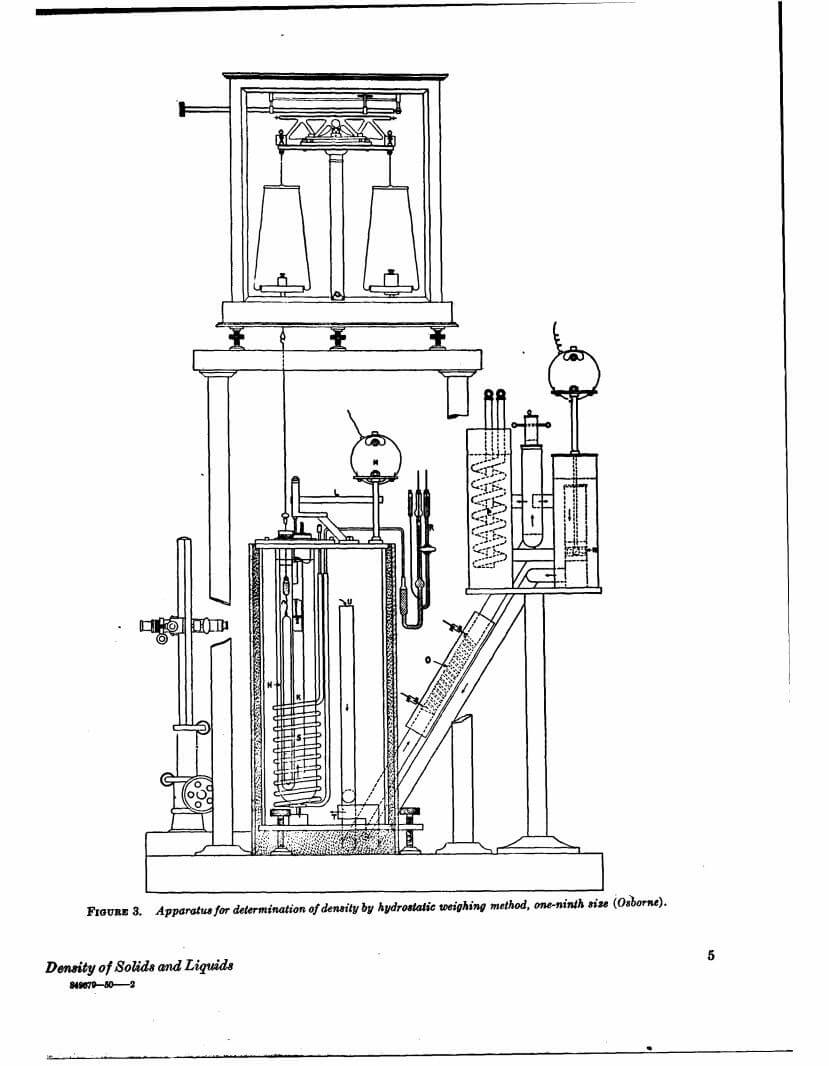
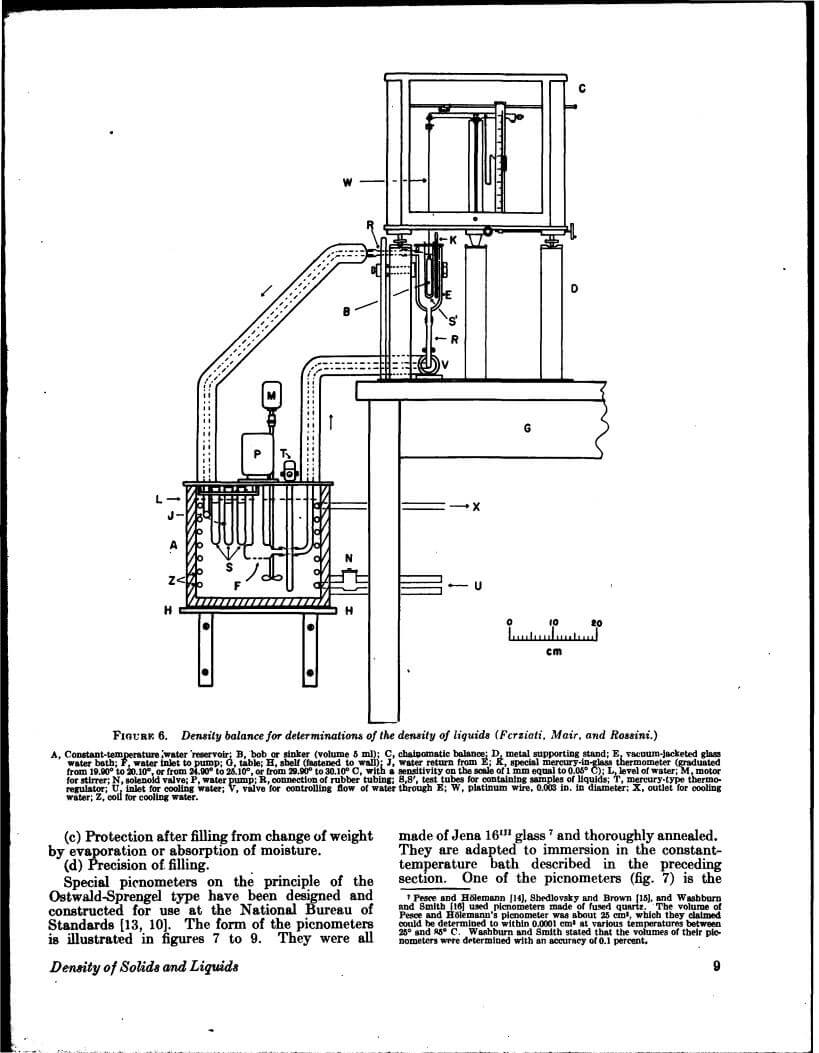
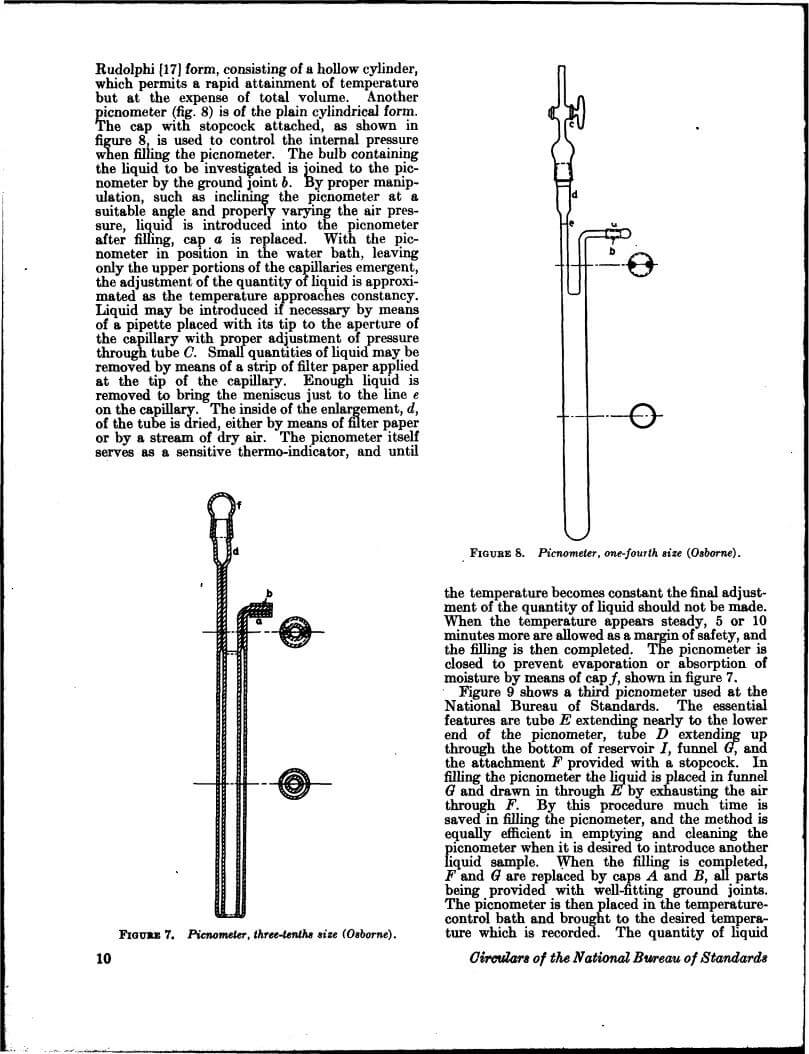
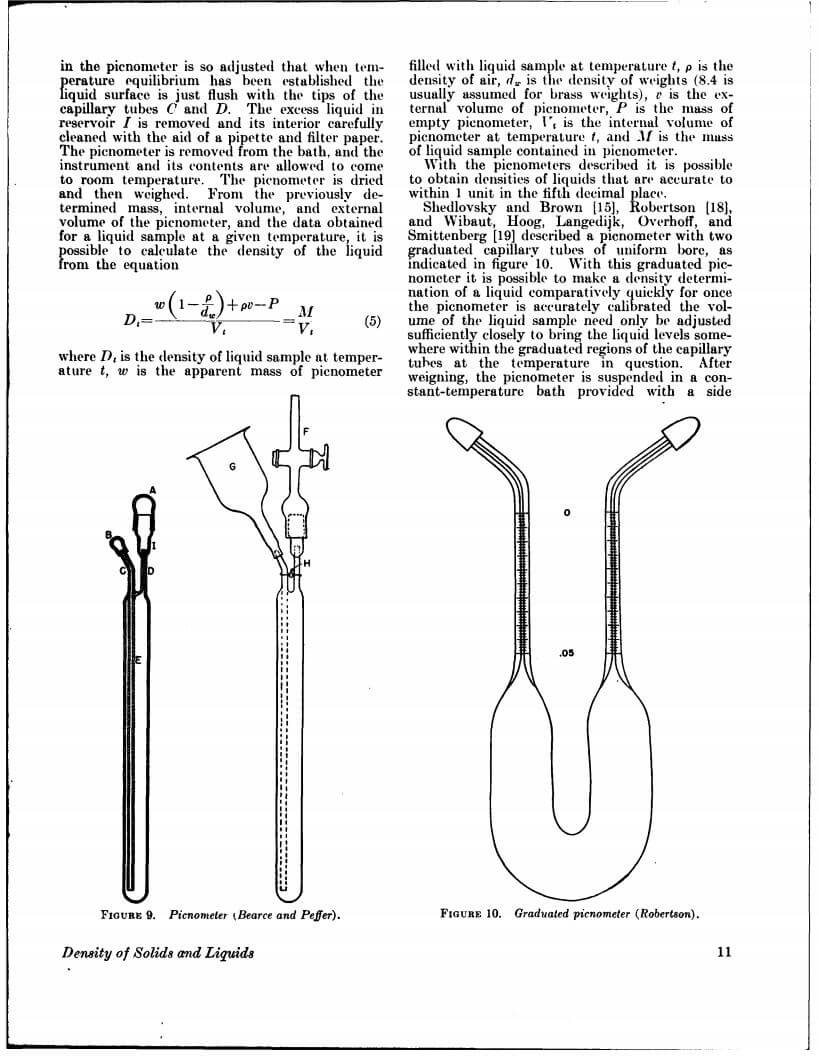
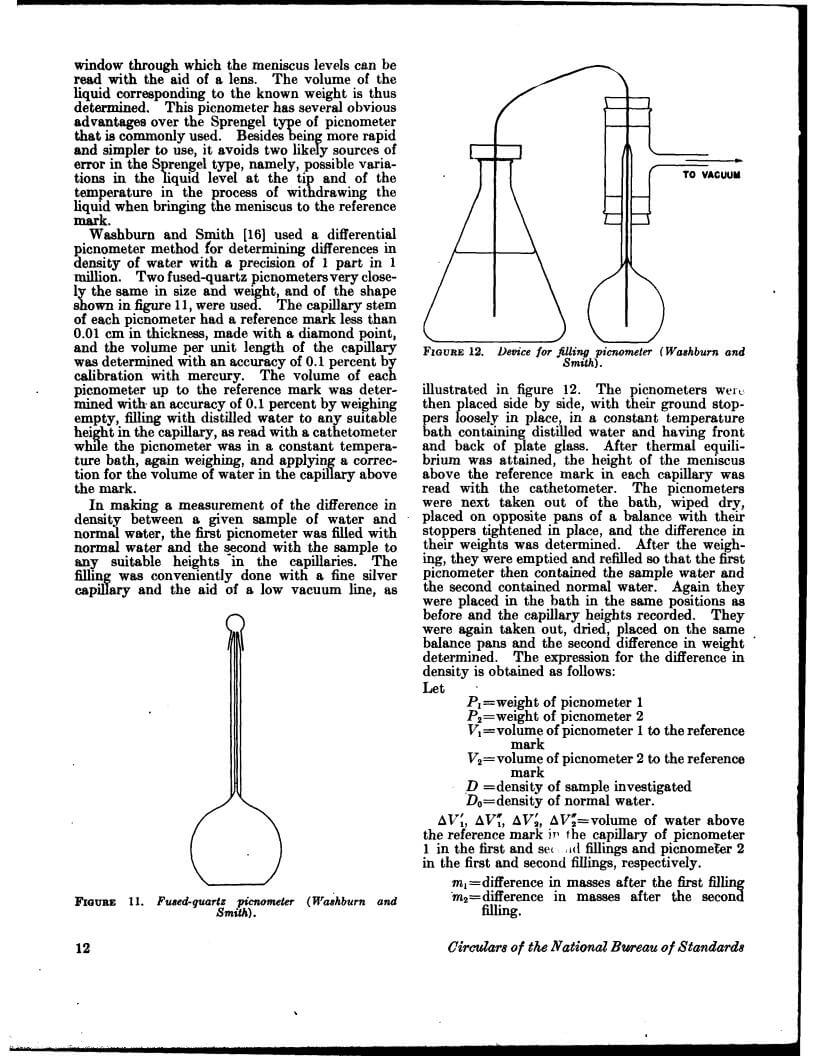
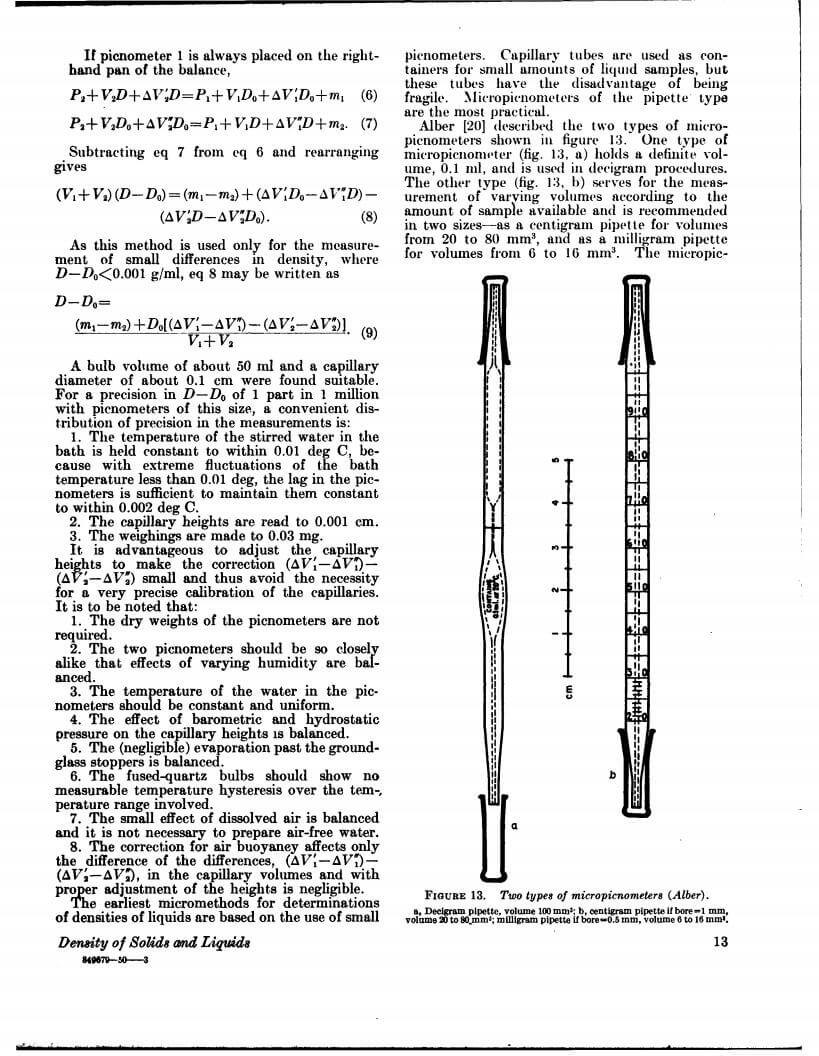
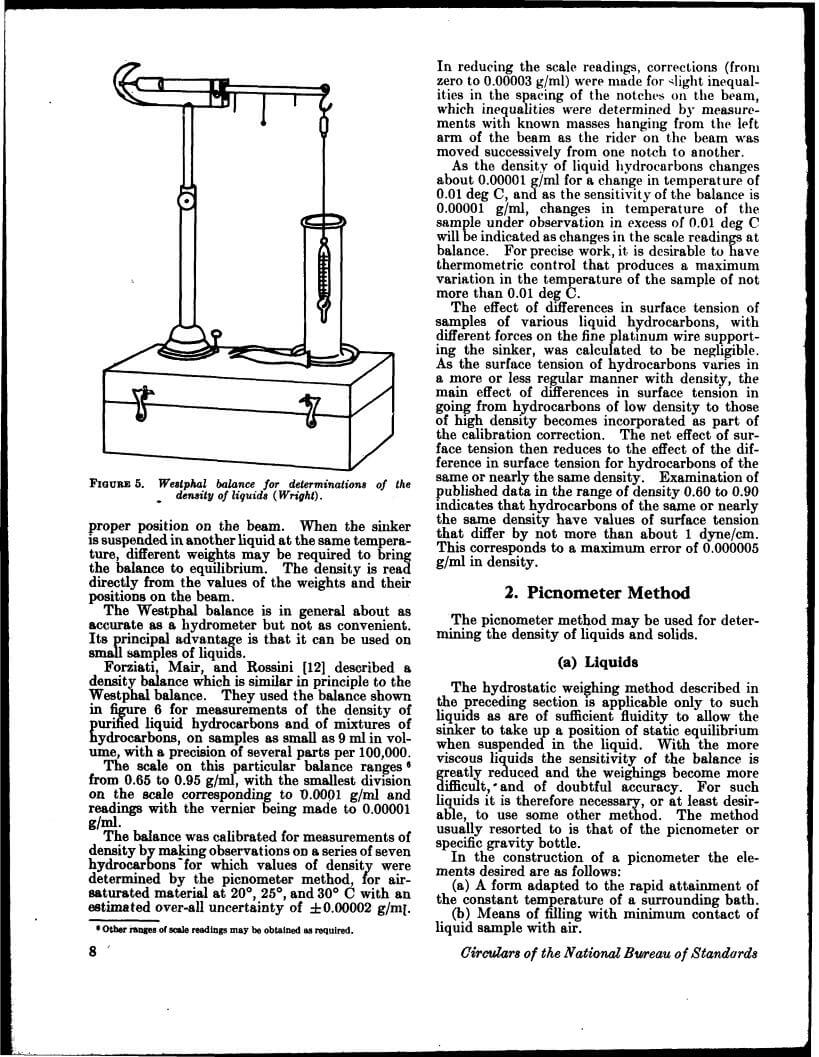
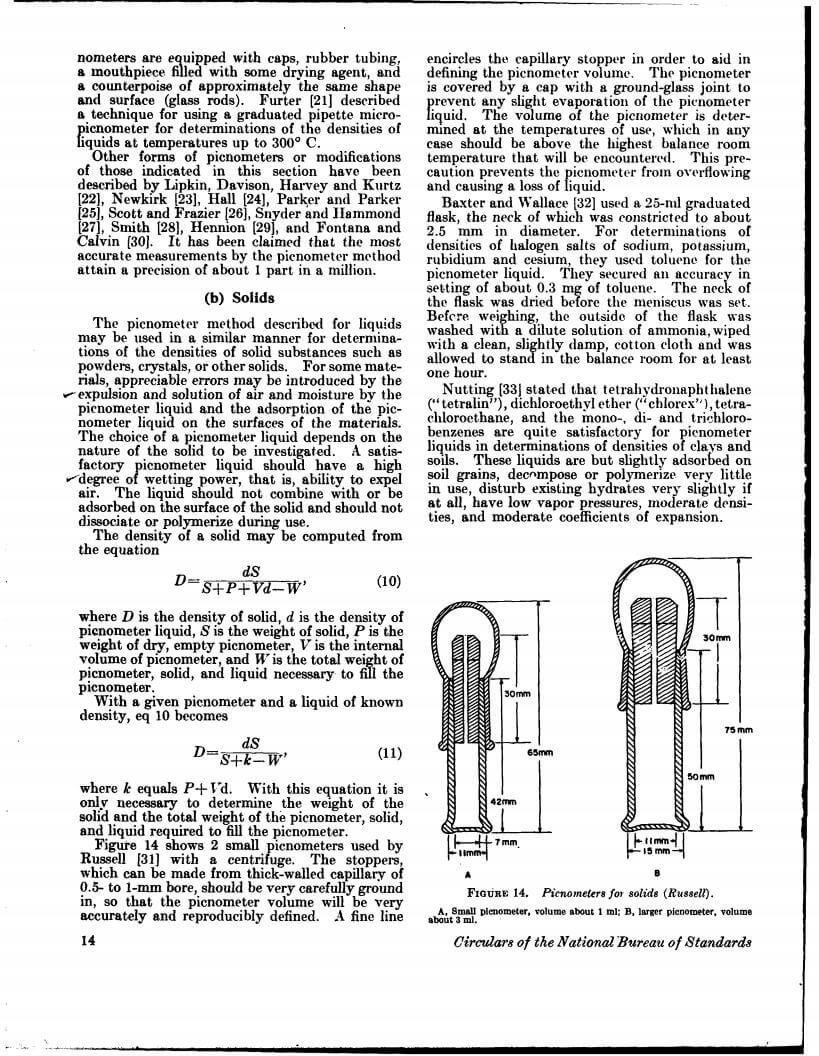
Density data may be used for obtaining relationships between density, chemical composition, thermal and mechanical treatments of materials, etc. This circular defines density and specific gravity, describes 11 methods (hydrostatic weighing, pycnometer, flotation, hydrometer, falling drop, balanced column, Boyle’s law. electromagnetic, elastic helix, ice calorimeter, and volumetric) for determinations of the densities of solids and liquids, and indicates the accuracy of various methods.
Density is a significant physical property of materials, and is important in science and industry. The extensive literature on density shows that determinations of the density of materials is a constant and recurrent problem. For example, density determinations may be used to identify materials, and to obtain relationships between density, chemical composition, thermal and mechanical treatments of materials, etc.
In any list of the physical properties of a substance, its density or specific gravity is usually included. If the values of the density of a substance are to be relied upon when obtained by different observers or by different methods, it is important that they be both accurate and comparable.
Density is the mass per unit volume and is usually expressed in grams per cubic centimeter (or per milliliter) at some definite temperature.
Specific gravity is an abstract number expressing the ratio of the weight of a certain volume of the material in question to the weight of the same volume of some other substance taken as standard at a stated temperature. Water is usually used as the standard substance. Both the temperature of the material and the temperature of the standard substance should be indicated.
Density and specific gravity, while fundamentally different, are nevertheless determined in the same way and may have the same numerical values. The two terms are often used synonymously as expressing the “heaviness” of a substance. Care must be taken, however, to state the units employed and the temperatures involved, otherwise there is danger of confusion and misinterpretation of results.