There are three methods for ascertaining the quality of gold, viz., by the touchstone and needles, by cupellation, and by parting or quartation.
The first is the process which, from its simplicity and convenience, is used by jewellers and others for learning at a glance the value of the articles which they buy and sell, and which are composed of gold, alloyed with different proportions of silver or copper; and this is ascertained by making a comparison between the specimen under examination and others of known value. The test is made upon a black silicious mineral called a touchstone, which is a variety of jasper known as the Lydian stone; and the specimens with which the alloys are compared, are points of gold alloyed with different, proportions of silver and copper, attached to small bars of silver, each marked with the number of carats of which the point is composed. These bars are connected together by a silver wire, as represented in Fig. 26.
 Sometimes the points are attached to a brass plate in the form of a star. The points on each are numbered from 6 to 24, being alloys from 6 to 24 carats fine.
Sometimes the points are attached to a brass plate in the form of a star. The points on each are numbered from 6 to 24, being alloys from 6 to 24 carats fine.
When an article is to be tested by this process, a corner of it is to be rubbed on the stone in such a manner as to form a mark about 1/32 of an inch wide, and ½ an inch long. A touch needle, resembling in colour the specimen under examination, is then rubbed in the same manner, so as to form a mark on the stone near to, and of the same size as the first. A drop of the acid mixture (which will be described shortly) is then put upon the two marks, and particular notice is to be taken of the difference in the marks produced by the acid. If the spot produced by the article examined, is brighter than that from the needle, this shows that it is better than the quality of that needle ; and if it is not as bright, that it is not so good. If no difference can be seen, this shows that the specimen examined is of the degree of fineness marked on the needle. After a few trials the operator will find the; needle corresponding with the specimen he wishes to test.
With a little experience, this process gives results, correct within one carat, which is sufficiently near for commercial purposes.
The test acid used with the above needles is composed of 98 parts nitric acid, of specific gravity, 1.34, 2 parts hydrochloric acid, sp. gr. 1.17, and 25 parts of distilled water. The
acid is kept for use in a low glass bottle, with a long tapering stopper, which nearly touches the bottom. (Fig. 27.) When used, a drop of the acid is to be taken out on the end of the stopper.
The process of cupellation is an ingenious method of separating foreign metals from silver or gold, by means of lead. To perform this operation, the furnace described in Chapter III. is well adapted. A vessel of refractory fire-clay, resembling a small oven, and called a muffle, (Fig. 28,) is put into the furnace, the front end of the muffle being supported by the lower opening, or door, in the front, and the back rests on a small bar of iron put through the two small doors at the side. A good charcoal fire is then to be made, with the front door closed; and when the muffle is bright red-hot, a perfectly dry cupel is put into it. Metallic lead, 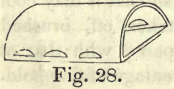 weighing about 2/3 of the weight of the cupel, is then put into it, and when the lead is fused, a portion of the gold to be assayed (about 1/14 of its weight) is to be wrapped in a thin piece of sheet lead, or blotting paper, and introduced into the melted metal. A good fire is now to be kept up, and the front door then opened so as to allow air to pass through the muffle, which causes the lead to oxydize, and
weighing about 2/3 of the weight of the cupel, is then put into it, and when the lead is fused, a portion of the gold to be assayed (about 1/14 of its weight) is to be wrapped in a thin piece of sheet lead, or blotting paper, and introduced into the melted metal. A good fire is now to be kept up, and the front door then opened so as to allow air to pass through the muffle, which causes the lead to oxydize, and
become converted into litharge, which dissolves the impurities in the alloy of gold, and is then absorbed by the porous cupel. Towards the last of the operation, the melted metal moves round rapidly, and becomes dull; suddenly the agitation ceases, the button becomes very brilliant and immovable. This is the end of the cupellation, and is called the brightening.
The cupel is then to be left in the muffle for a few minutes, and drawn gradually towards the mouth, before it is taken out, so that it may cool slowly. The button is then taken off, brushed and weighed. Its weight compared with that of the alloy used, gives the per centage of fine gold.
With practice and good management cupellation becomes easy, and is quickly performed; and although it does not give results which are exactly correct, it is sufficiently so for most purposes, and it is the process generally in use by goldsmiths for assaying gold and silver.
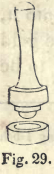
The cupels used for this process are made of powdered bone-ash, as follows : 4 lbs. of the ash are well mixed with 1 lb. of beer; the bottom ring of a cupel mould (Fig. 29) is then filled with the mixture, and the handle pressed down; this gives form to the cupel; the ring is then turned over, and the cupel removed by pressing on the bottom. When dried and ignited it is ready for use.
The only process which is absolutely correct for assaying gold, is that, in use at the mint, and is called parting, or quartation.
Small portions of the specimen to be assayed are cut off from different parts of the ingot, so as to get a fair sample of it. Twelve grains of the metal are then carefully weighed on a delicate balance (and for making a correct assay a delicate balance is necessary, together with a set of accurate assay weights, made for the purpose.)
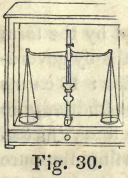 The balance represented in Fig. 30 is the kind employed. It is enclosed in a glass case, with a sliding-door, to keep it from dust and currents of air, while weighing. The set of platina weights contains 12, 6, 5, 3, 2, 1, ½, ¼, 1/8, 1/16, 1/32, grains. These are made very carefully from the standard used in the mint.
The balance represented in Fig. 30 is the kind employed. It is enclosed in a glass case, with a sliding-door, to keep it from dust and currents of air, while weighing. The set of platina weights contains 12, 6, 5, 3, 2, 1, ½, ¼, 1/8, 1/16, 1/32, grains. These are made very carefully from the standard used in the mint.
Having previously ascertained the comparative value of the alloy, by means of the touchstone, 12grs. are carefully weighed and mixed with a proportion of silver, which, added to that already in the alloy, shall make the proportion of silver equal 2½ times the weight of the fine gold. The mixture of gold and silver is then cupelled with twice its weight of lead. The resulting button is flattened by a hammer, then rolled out into a thin ribbon, about 2 inches long. This is to be wound on a quill or pencil, so as to form a thin spiral, which is to be put into a small flask, of the capacity of 3 ounces. 2oz. of nitric acid, of 22°, (sp. gr., 1.16) is then poured on, and the mixture heated for 3 or 4 minutes. Then replace the first acid with 2oz. of that which is 32°, or stronger, (sp. gr. 1.26,) boil ten minutes, then replace with fresh acid of 32°., and boil ten minutes longer. The residue of gold, which is of a dull red colour, is to be washed well in the flask, and then transferred to a small crucible, dried and heated without fusion. It is then to be carefully weighed on the assay balance.
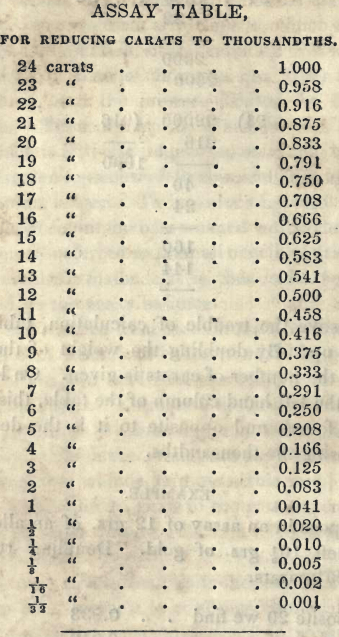
The standard of gold is expressed by the term carat in commerce, and in thousandths by government. Pure gold is 24 carats or 24/24 fine : 18 carats fine is 18/24 pure, &c. Expressed in thousandths,pure gold is 1000/1000 fine: 18 carats fine is therefore 750/100, &c.
Example. If in the process of parting, the pure gold remaining weighs 11 grains, this is 11/12 or 22/24 pure. That is, in 24 parts of the sample assayed, there is 22 parts of pure gold. It is consequently 22 carats fine.
If we wish to express this in thousandths, in the absence of a table, it is easily performed by the rule of proportion: as 22/24 is a whole, so is 1000/1000. In this example we have an alloy 22 carats fine; now the following statement expresses the thousandths : as 24 is to 1000 so is 22.
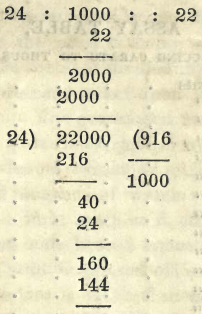
To save the trouble of calculation, tables are drawn up. By doubling the weight of the pure assay, the number of carats is given. On looking down the left hand column of the table, this number is found, and opposite to it is the decimals expressing the thousandths.
EXAMPLE.
Suppose in an assay of 12 grs. of an alloy, we have left 10i 1/8 grs. of gold. Doubling 10 1/8, we have 20¼ carats.
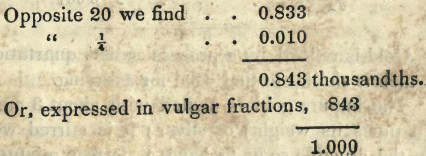
Gold is refined by a process called quartation which is similar to that used for assaying.
The impure gold is melted with about 3 parts (3 times its weight) of silver; it is stirred well in the crucible so as to have a perfect mixture; and this is poured into a large dish of water, which is kept in motion by an assistant, who stirs it with a stick while the alloy is being poured in. By this process the metal is divided into small lumps, which are more easy to act upon with acid than when in mass. The granulated metal is then put into a large glass flask, and heated on a sand bath, with twice its weight of nitric acid diluted with an equal measure of water. When the action ceases a little more acid, stronger than the first, is put on and heated again till there is no action. The acid is poured off, and the gold remaining in powder is washed several times, till a portion which comes off will give no precipitate when mixed with a solution of common salt.
The gold is then taken out, dried, melted with a little borax, and poured into water, or an ingot mould. This is refined gold.
The solution in nitric acid is diluted with water, to form 2 or three times its original measure. Plates of copper are then immersed in it, and allowed to remain 24 hours or more, till the silver is all precipitated, and the solution no longer contains silver. This is ascertained by taking out a small portion of the blue liquid, and if it gives no precipitate on the addition of a solution of common salt, it contains no more silver. It is then to be washed repeatedly with water till the blue colour is entirely removed. Dilute sulphuric acid is then added, and allowed to cover it for a few hours. It is then washed again, till the washings are tasteless. The gray powder of silver is then dried and melted with a little borax. The product is refined silver.
