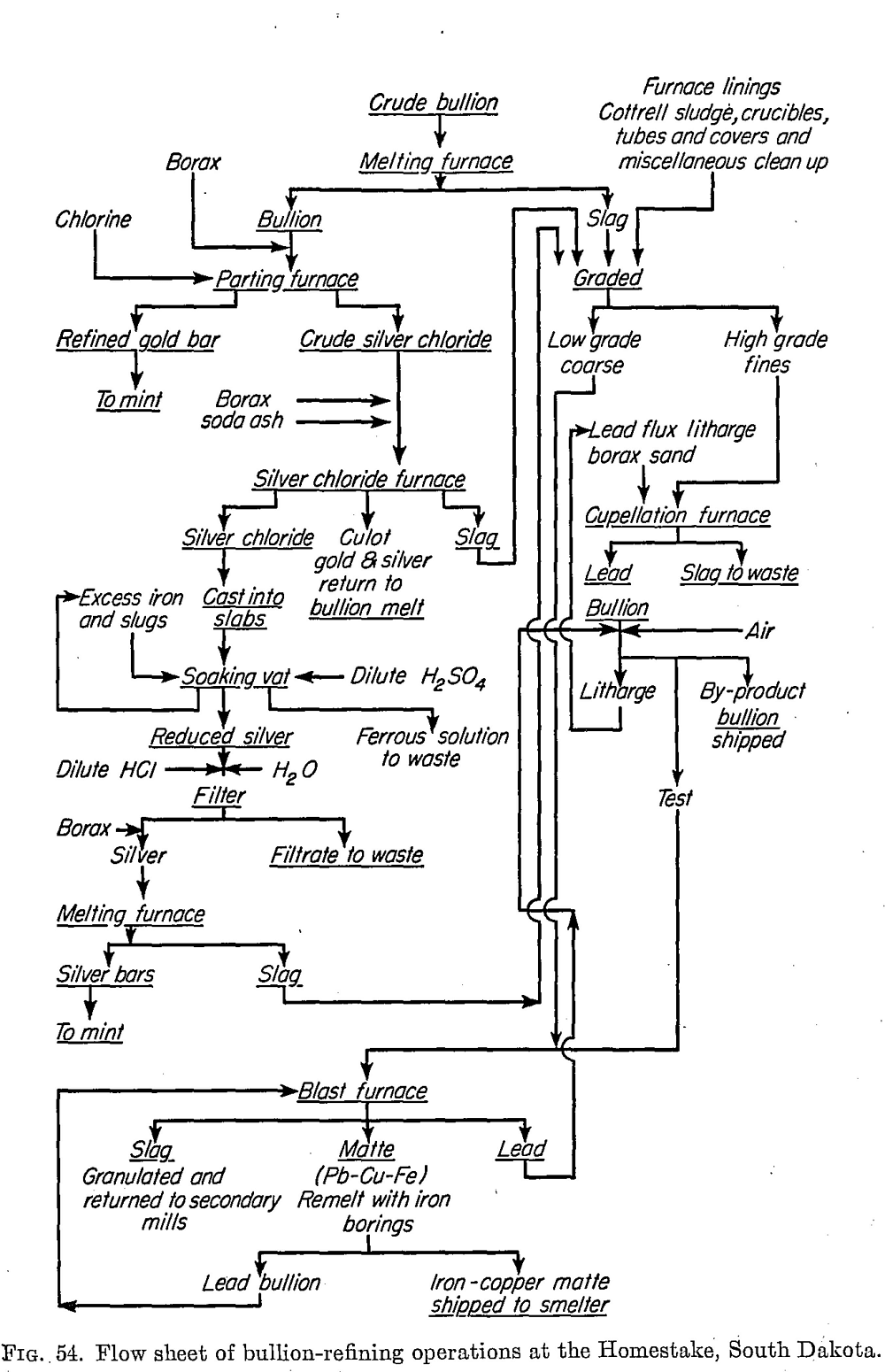
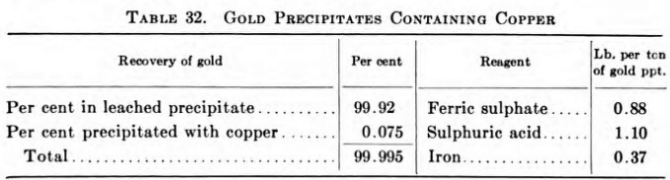
A paper with this title by Norman Hedley and J. J. Kress, Trans. 48, CJ.M. and M.,252-258, 1945, describes a method evolved at the Ore Dressing Laboratory of the American Cyanamid Company for treating gold precipitates containing copper. This comprises treating the precipitates with acidified ferric sulphate, followed by precipitation of the dissolved copper and any dissolved gold by metallic iron to give a product analyzing 60 per cent copper. In the case of a gold precipitate carrying 10.06 per cent Au; 0.56 per cent Ag and 26.21 per cent Cu, a final product carrying 40.32 per cent An, 2.25 per cent Ag, and 8.27 per cent Cu was made, representing a 75.05 per cent reduction in weight.
The advantages of this scheme are listed as:
- The weight and bulk of the precipitate would be reduced and then less flux and fuel would be required for melting.
- Special reagents would not be required to flux the copper, with the result that less corrosive slags would be formed and the life of the crucibles would be prolonged accordingly.
- Large quantities of slags and material carrying various amounts of gold and requiring special treatment would not be formed.
- Preliminary roasting would not be necessary, and possible losses due to dusting and handling would be avoided.
- The copper would be recovered in a high-grade product and credit for it and for the contained gold would be obtained at the smelter.
