The reduction of iron from the ferric to the ferrous state prior to its titration by permanganate or dichromate may give erroneous results in the presence of other reducible metals such as titanium. Stannous chloride and metallic zinc both reduce tetravalent titanium to a lower form, and so increase the consumption of the oxidizing standard solution. Sulphuretted hydrogen and sulphurous acid do not reduce titanium, but are slow in action and troublesome to manipulate.
Sulphuretted hydrogen is considered to be the best reducing agent for iron in the presence of titanium. In the method to be described the advantages of the H2S reduction are retained without the disadvantages of using the gas. The reduction is carried out by the action of a metallic sulphide having a solubility product of such a value that the sulphide ions are utilized as fast as they are formed, and but little free sulphuretted hydrogen is given off.
Cadmium sulphide was the first to be tried, but its solubility product (3.6 x 10 — 29) was apparently too low. The reduction was satisfactory, but the excess was very difficult to remove. Zinc sulphide (S.P. = 1.2 x 10 — 23) was found to be quite satisfactory in both respects. The zinc sulphide is preferably used in the form of an emulsion prepared from pure zinc sulphate as follows:—About . 100 grm. of ZnSO4 7H2O is dissolved in 2 litres of water. H2S is passed until no further precipitate is obtained, the acid formed being barely neutralized from time to time by the addition of ammonia. The precipitation of the sulphide in a slightly acid solution gives a finer emulsion than that prepared in an alkaline solution, and any iron present remains dissolved. The precipitate is allowed to settle and the clear liquid decanted. The precipitate is then shaken up with more water, acidified with a little sulphuric acid, allowed to settle, and the liquid decanted as before. Water is added to bring the volume to about 2000 cc. This emulsion keeps indefinitely, and only requires shaking to be ready for use.
PROCEDURE FOR PERMANGANATE TITRATION
The Sulphuric acid solution containing the iron to be titrated is placed in a short-necked flask and heated to boiling. Sufficient sulphide emulsion to give a decided milkiness is added slowly, and the boiling continued until no reaction is obtained with a drop of thiocyanate on a spot-plate. If the concentration of sulphuric acid is hot too great, complete reduction is obtained very quickly, not the slightest colour being given with sulphocyanide.
If the sulphur liberated by the reaction is brownish in colour, small amounts of heavy metal sulphides are present, and should be filtered off before removing the excess of zinc sulphide. If, however, the residue is white or faintly yellow, some dilute sulphuric acid and a piece of pure, white marble is added, and the solution boiled vigorously until the sulphur becomes granular and the solution quite clear. By this time the slight excess of sulphuretted hydrogen should have been removed, but it is preferable to make quite sure by testing the vapour with lead acetate paper. The flask is then cooled quickly under the tap, and the cold solution titrated in the usual manner with standard permanganate solution.
A blank titration, using the same amounts of acid, zinc sulphide, and marble, should be made. Not more than 0.1 cc. of decinormal permanganate should be required.
PROCEDURE FOR DICHROMATE TITRATION
If the solution containing the iron is strongly acidified with hydrochloric acid, the greater part of the acid should be neutralized before reducing with the sulphide. The emulsion is added to the boiling solution as before, but the progress of the reduction is indicated by the disappearance of the yellow colour of the ferric chloride. On account of the greater solubility of the marble in the hydrochloric acid, care must be taken that the ebullition does not become too vigorous. A stream of carbon dioxide from a Kipp apparatus may be used instead of the marble, or it may be dispensed with since the titration can be carried out hot there is little chance of oxidation by the air taking place. The solution is titrated with decinormal potassium dichromate, using a somewhat weaker ferricyanide solution than usual as indicator. The comparatively small amount of zinc in solution does not appear to interfere with action of the indicator.
A blank experiment should, of course, be conducted, as in the case of the permanganate titration.
TITRATION OF A FERRIC SOLUTION AND THE EFFECT OF TITANIUM
A solution of ferric sulphate acidified with sulphuric acid was prepared. The amount of iron in this was determined by measuring out 50 cc. with a standard pipette and precipitating the iron as hydroxide with ammonia after adding a little paper pulp. The precipitate was filtered, washed, ignited strongly, and weighed as Fe2O3. The weight was 0.1482 grm., which corresponds to 0.1037 grm. of iron.
The following are the results of titrations of this solution, taking. 50 cc. and reducing with 20 cc. of the zinc sulphide emulsion :—
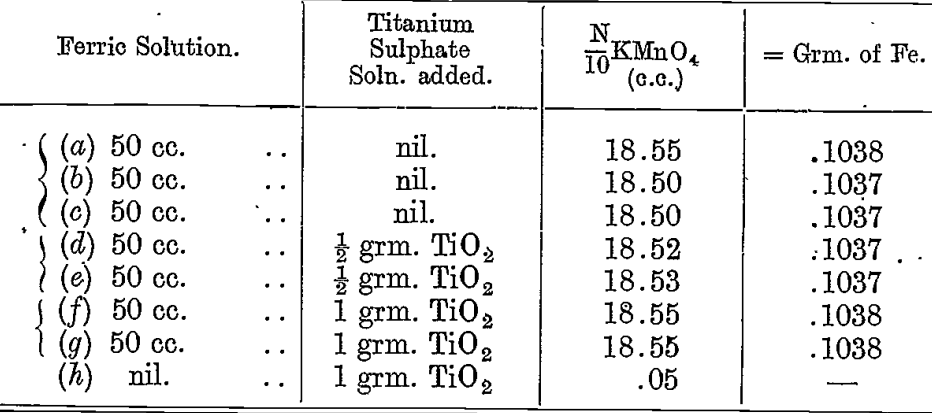
A trace of iron was found in the titanium solution. This evidently accounts for the slight increase when the titanium was added.
COMPARISON OF METHODS OF REDUCTION
A solution of pure ferric alum was prepared. This was titrated with KMnO4 after reduction with (a) electrolytic zinc and (b) zinc sulphide. It was also titrated with dichromate after reducing with stannous chloride and zinc sulphide respectively.
50 cc. of the solution was taken in each case, and the iron was determined in the same amount by precipitation; 0.2099 grm. of Fe2O3 were obtained, equivalent to .1469 grm. Fe.
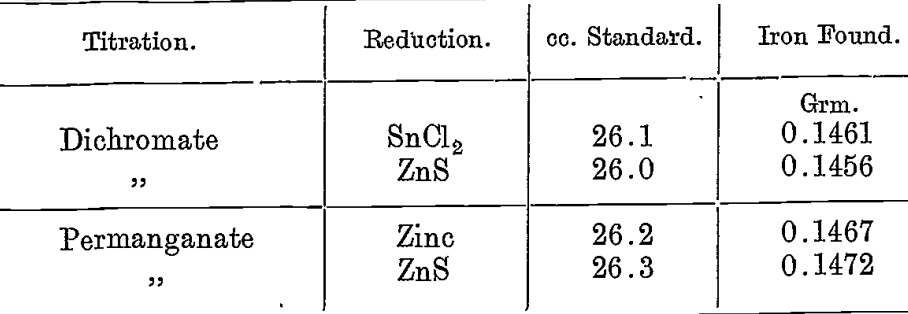
For these titrations the standard solutions were both checked by standard thiosulphate, the latter being compared with decinormal hydrochloric acid by means of the iodide-iodate reaction. The hydrochloric acid was standardized by calc-spar.
A sample of haematite iron ore was handed to eighteen students for analysis, using both zinc and zinc sulphide reduction. The averages of their reports were :—Zinc reduction, 55.3 per cent, iron; and zinc sulphide, 55.4 per cent.
A standard sample of titaniferous iron ore was analyzed by this method. It contained 60.49 % of iron and considerable titanium; 0.25 grm. was taken in duplicate, requiring (a) 27.10 cc. and (b) 27.08 cc. of decinormal permanganate. These are equal to (a) 60.48 and (b) 60.43 % of iron respectively, after allowing 0.1 cc. for the blank.
The foregoing results show that the reduction of iron with zinc sulphide is at least as accurate as the older reduction methods. The reagent can readily be prepared in a state of purity, and its efficiency does not alter on keeping. The method provides a rapid and reliable procedure, which is of particular value to the chemist who prefers the permanganate, but who uses the hitherto more expeditious dichromate method for the determination of iron even when some titanium is present.
