 For a number of years I have used for the commercial wet assay of lead generally the ammonium molybdate, and occasionally the ferrocyanide method. These well-known methods need no detailed description here. In the ore-selling and ore-buying establishments of the West, 90%, of all wet lead-assays are made by one or the other, and at least nine-tenths of this proportion by the molybdate method.
For a number of years I have used for the commercial wet assay of lead generally the ammonium molybdate, and occasionally the ferrocyanide method. These well-known methods need no detailed description here. In the ore-selling and ore-buying establishments of the West, 90%, of all wet lead-assays are made by one or the other, and at least nine-tenths of this proportion by the molybdate method.
A procedure so well-established must have merit; and, in fact, the ammonium molybdate method, when applied to siliceous ores or products of fairly high grade, has proved itself both rapid and accurate to a satisfactory degree. Yet those who use it most constantly clearly recognize its weaknesses in certain respects and under certain conditions; and I believe that a method free from such weaknesses would be welcomed by analysts. In this belief, I submit the results of my laboratory experiments, covering a period of nine months, during which a method was developed by which more than 2,000 wet lead-assays were made on various ores and under purposely varied conditions, in order to determine the conditions necessary for accuracy, and to test the applicability of the method to all kinds of pure and impure lead-ores and products.
One weakness of the ammonium molybdate method is the end-reaction with the indicator—a freshly-prepared solution of tannin. The usual practice is to titrate at boiling-heat, and, for a high percentage of metal, to make a second boiling after getting a faint end-reaction, and then to finish to a complete end-tint. The correction for the indicator is, to some extent, affected by the personal equation involved in the operator’s conception of a perceptible yellow tint, and varies among different analysts from 0.3 to 0.5 cc. of a standard solution of which 1 cc. equals 10 mg. of lead. It is evident that while this is quite permissible with fair-grade ores (and at the same time have different operators check quite closely enough for commercial purposes), it is an entirely different matter in dealing with tailings containing, say, 0.3 per cent, of lead, in which the variation in the correction used for the indicator would represent practically the total quantity of lead present.
A somewhat extreme instance showing this weakness came under my notice a few months ago. A series of samples of siliceous tailings, carrying from 0.3 to 0.5 per cent, of lead, were sent for checking-purposes to a leading umpire-assaying establishment in the West, with the statement that they were tailings for wet lead-assay. The report was, for about half of the lot, “trace;” for the remainder, “ none.” The analyst wrote us that the ammonium molybdate method had been used, and that, although he could see fine galena in the tailings, and could even pan it out, he had found, on assaying, that the number of drops of molybdate solution necessary to react with the tannin was in each case no more than the correction-amount for the indicator; so that he was forced to make the report as he did.
Assuming that the amount of ammonium acetate used, and the bulk of solution for titration, were at the minimum limits for such fractional percentages, it is probable that if, in these cases, the analyst had given his flasks a prolonged boiling after adding the first few drops of molybdate solution, he would have found that his end-tint had faded sufficiently to accommodate a few additional drops of the standard solution. This would have given him, above the indicator-requirements, a small fraction which he could then have labeled “lead.” A result, however, which has to be squeezed out by such labored and uncertain efforts is neither gratifying to the analyst nor really valuable to his client.
Furthermore, the lead molybdate precipitate being white, and the practice being common among analysts of not pouring the ammonium acetate solution through the sulphates on the filter, but of depositing filter and all in the original flask, digesting therein with ammonium acetate, and then titrating, it follows that, with only slight traces of lead present, there is no proof positive to the eye that the few drops of molybdate added really found any lead to precipitate, the solution being turbid from the slimes of the filter. The analyst is in doubt whether to report, say, 0.2 per cent, of lead, and chance it; to compromise on a “trace;” or to make a clean sweep and say “ none.” This hypothetical case may be considered by many as overdrawn, but I know from personal experience whereof I speak.
The other main and commonly-occurring weakness of the molybdate method is shown in dealing with ores containing large quantities of lime. Should the percentage of lead be fairly large, say 5 per cent, or more, the molybdate method does very well; but with small and fractional percentages of lead, all the above-cited troubles occur and are aggravated by the tedium of washing the bulky precipitate of pasty calcium sulphate derived from the sulphuric-acid evaporation. Ores of this class are common in southeastern Missouri; and, although remarkably pure in the sense of containing little else than galena in slightly siliceous dolomite, they have given much trouble to analysts who have to look for fractional percentages of lead in such material.
Some years ago, having experienced difficulties somewhat analogous to those above mentioned, I gave up the use of the molybdate method on material containing less than 1 per cent, of lead, and for some time, employed a method of precipitating those small amounts of lead from the hot, filtered ammonium acetate solution (after it had been acidified with hydrochloric acid), on a strip of pure aluminum free from silicon. The precipitated lead was removed from the aluminum strip by rubbing, washed, dried at 110° C., between filter-papers, and weighed as metallic lead. This method is tedious if many determinations are to be made, and it possesses several disadvantages, not the least being that, when the quantity of lead present exceeds from 20 to 30 mg., small portions of the lead- film are liable to become detached before the operation is completed, and, floating around in the acid solution, to be slowly dissolved.
The result of many trials led me to experiment with the precipitation of lead as chromate under various conditions; and this method, as finally elaborated and tested, has proved so rapid and satisfactory in every way that I have discarded all other methods, and use this not only for small percentages of lead, but for all wet lead-assays, on whatever material.
The insolubility of lead chromate in water, and in dilute acetic acid, is well-known, and the gravimetric method of estimation by chromate precipitation has been in use to some extent for years. Modifications, looking to the development of this method into a volumetric one, have also been published; but the fact remains that, at the present time, the volumetric chromate method as a commercial wet lead-method is practically unknown.
Taking advantage of the reactions of lead chromate, together with such literature as bore upon the subject, preliminary trials were made. A solution of normal potassium chromate was generally used, although dichromate answers nearly as well, the normal chromates being converted to dichromates on addition of acids; but as I have generally used the normal chromate for the precipitating solution, and as its equations are somewhat simpler, I will consider it here.
(1) K2Cr04 + Pb(C2H302)2 = PbCrO4 + 2KC2H3O2
The potassium chromate solution is added in slight excess of the quantity necessary to precipitate all of the lead; the lead chromate is separated by filtration and washing; the filtrate containing the excess of chromate is acidified with about 25 cc. of dilute hydrochloric acid (1:1); a small crystal of potassium iodide (about 0.5 g.) is then added; and the liberated iodine is titrated with a standard solution of sodium hyposulphite in the usual manner, adding the “hypo” until the brown color of iodine has almost disappeared. Then a few drops of starch paste are added, and the titration is continued until the blue color has just disappeared, and a clear solution remains. The standard hyposulphite solution is preferably made of such a strength that 1 cc. will equal 0.5 cc. of chromate solution, in order to afford an easy calculation of the back-titration, in terms of chromate. The equations of the back-titration are:
(2) 2K2Cr04 + 6KI + 16HCl = 10KCl + 2CrCl3 + 8H20 + 3I2
(3) 6Na2S203.5H20 + 3I2 = 6NaI + 3Na2S406
Equation (1) shows that, of a solution of K2Cr04 of 9.396, or approximately 9.4 g. per liter, 1 cc. will precipitate 10 mg. of lead. If the dichromate solution be used, it should contain 7.12 g. to the liter, in order to give the same strength. Equations (2) and (3) show that, of a solution of “hypo” of 36 g. of the pure crystallized salt to the liter (or 18 g. to the liter, if the more desirable half-strength be used), 1 cc. equals 1 cc. of the chromate standard. This is very nearly the strength of the standard “hypo” in common use for the iodide assay for copper, and if only a small number of wet lead-assays are made daily, it may be more convenient to use the copper “hypo” solution for the back-titration of the chromate; in the latter case the factor necessary to convert the copper “hypo” solution to terms of chromate is about 0.54, the exact factor being readily ascertainable by standardizing the one solution against the other.
Numerous trials were made by taking weighed quantities of pure lead sulphate, dissolving them in slightly acid ammonium acetate, diluting slightly, running-in a slight excess of the standard chromate solution, and, after filtering and washing, determining the excess by “hypo” titration. These tests have shown that the precipitation of the lead is in strict quantitative accordance with Equation (1), so that, in preparing the chromate solution with pure salt, its theoretical value will be found to check exactly when standardized against pure lead sulphate under the conditions of the assay.
The accuracy of the standard having been determined against pure lead in solution as lead acetate, extended experiments were made to determine the possible interference of other elements, testing them in successive experiments by adding a different metal in the form of its convenient salt to each of a series of flasks, containing weighed quantities of pure lead sulphate; then digesting with nitric and sulphuric acids to complete white fumes; cooling; diluting with water; reheating in order to dissolve soluble sulphates; filtering; washing; returning the filter and its sulphates to the original flask; adding slightly acid ammonium acetate; and digesting a few minutes until the lead sulphate had completely passed into solution,—all in the usual manner of wet lead-assays. These respective solutions were then diluted slightly with cold water, and titrated with the standard chromate and “hypo” solutions, as described for the pure-lead standard.
These trials were made repeatedly, not only on artificially prepared charges, but later, on actual mixed ores, covering all conditions. The only possibly interfering elements are those which under these conditions do not go into solution readily as sulphates, and of these the calcium-barium-strontium series, tungsten and antimony are the only ones commonly occurring. Lime, although troublesome to wash, causes no interference in the results for lead, through any lime that might be in solution in acid ammonium acetate; since calcium chromate is freely soluble. Barium and strontium salts remain insoluble as sulphates, and tungsten yields the insoluble tungstic oxide, hence the compounds of these three elements cause no interference. Antimony, when present in considerable quantity, caused the results to be slightly low. This was found to be due to imperfect solution of the lead sulphate in slightly acid ammonium acetate in the presence of the residual mixture of antimony oxides, varying from Sb203 to Sb204 and Sb2O5, depending on conditions. This difficulty was rectified by prolonging the digestion of the filter and its contents, and using slightly ammoniacal ammonium acetate for the digestion, in order to offset the acid nature of the antimony residues. The solution was then slightly acidified by the addition of acetic acid after dilution, and before adding the standard chromate. In none of these cases is it necessary after digesting with ammonium acetate to make a second filtration before adding the chromate; the flask with its contents and the remains of the filter, when cooled and diluted, are ready for the precipitation by chromate.
In adding the standard chromate solution, it is desirable, if the lead-content of the assay be known approximately, to add only a few cubic centimeters in excess, in order to minimize the washing and a lengthy back-titration. The filtration may be made directly after adding the chromate solution, using an ordinary 11-cm. filter of S. & S. No. 597, or No. 604, or any other fairly rapid and close paper; the lint present in the flask from the partial disintegration of the original sulphate filter, serving to check any tendency of the lead chromate to pass through. For the reason that the total bulk of the solution is only about two funnelfuls, and the precipitate one that is readily washed, this operation consumes but little time, and a 250-cc. flask serves to accommodate easily the filtrate, washings, and the hydrochloric acid necessary to liberate the iodine in the back-titration.
In dealing with fair percentages of lead, the exact quantities of ammonium acetate and of water for dilution are not of great importance; but when only about 1 per cent, of lead, or less, is present, and it is desired to make the filtration for the back-titration directly after adding the chromate, it is necessary to keep the bulk of the strong ammonium acetate solution used, within 10 cc., and to dilute with cold water to a bulk not exceeding 50 cc., before adding the chromate. As has been proved by careful experiments in this laboratory, in passing far beyond these limits, the solution seems to be on the one hand too strong in ammonium acetate for the small quantity of lead present, and, on the other hand, too dilute in milligrams of lead per cubic centimeter for the complete instantaneous precipitation of the lead chromate, although, if the time can be afforded, the precipitation becomes complete on standing a few hours, even when greatly beyond the limits given. From adding a large surplus of chromate solution, complete precipitation results at once, but such a surplus for back-titration is not desirable in dealing with small or fractional percentages of lead. If desired, however, an excess of chromate solution can be added, and, after filtering, instead of a back-titration (except in the presence of antimony), the funnel with its filter may be placed over a clean flask, and hot dilute HCl (1:1) added to the original precipitation-flask, in order to dissolve the lead chromate precipitate therein contained, taking care that the remains of the sulphate filter do not retain any undissolved precipitate. This hydrochloric acid is then poured through the funnel to dissolve the portion on its filter, washing both flask and filter with warm water. This hydrochloric acid solution of the lead chromate, after adding a little potassium iodide, is ready for a direct titration with “hypo” solution in the same manner as that for the back-titration, and the results are perfectly concordant with those calculated from the back-titration.
The bulky residues present from the large ore-charges usually used in low-grade assays render this modification tedious, owing to the time necessary in washing these residues free from the excess chromate solution, and it will be found more satisfactory to use the back-titration with the slight surplus of chromate solution.
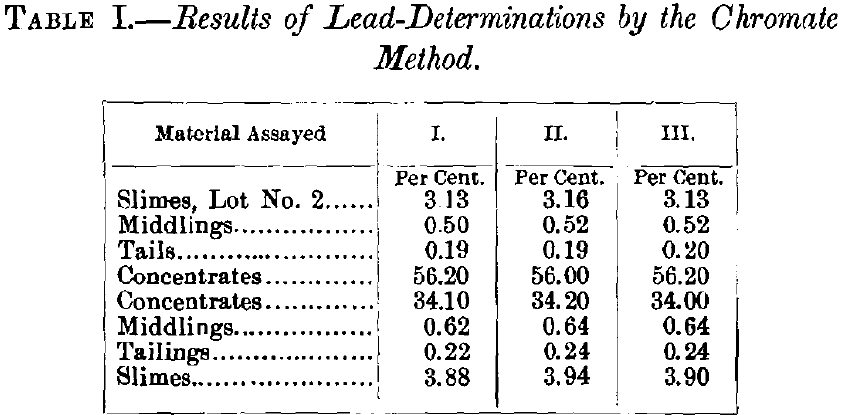
Within the past few months I have had occasion to make several hundred wet lead-assays of ores and mill-products from southeastern Missouri of an average composition of about CaC03, 45; MgC03, 30; SiO,2, 8; Fe, 5; and lead, from 0.2 to 5 per cent, depending on the product. These assays were made in triplicate, by the chromate method above described, and very satisfactory accordance was obtained in each set. A triplicate series of the results obtained for the lead-content, taken at random from the assay-files, is given in Table I.
In practice the method takes about the same length of time as the molybdate method on the same type of ore.
After making some scores of assays in this manner on these heavy lime-ores, it occurred to me that possibly the sulphuric acid feature might be omitted entirely, thus avoiding the tedious washing of the resultant bulky masses of calcium sulphate, without affecting the accuracy of the results. Experiments resulted in the following short-cut method for heavy lime-ores. To the ore-charge of from 1 to 5 g. in a 250-cc. flask add from 3 to 5 cc. of strong nitric acid and 15 cc. of strong hydrochloric acid; digest until everything is in solution, and the excess of acid has been reduced to about 8 cc. The whole operation on the hot plate requires but 15 minutes. The flask is then removed, and slightly dilute ammonia is added slowly in small excess, the neutralizing action being sufficiently vigorous to render the contents of the flask quite hot. Acetic acid of 80 per cent, strength is then added slowly, the flask being shaken vigorously, until its smell indicates a decided excess of acetic acid. Then 5 cc. of strong ammonium acetate is added to insure the solution of any lead compounds remaining undissolved by the ammonium acetate already formed in the flask.
If the ore contains no antimony, or separated gelatinous silica, and if the siliceous residue in the bottom of the flask is only in slight amount (as is usual with heavy lime-ores or with concentrates), add to the hot, undiluted and unfiltered solution, an excess—about 10 cc.—of a 10 per cent, chromate solution. Under these conditions, the bulk of the contents of the flask will not exceed 50 cc.; and, after shaking and letting the precipitated lead chromate settle for about 5 min., the contents are filtered through a 11-cm. filter of any fairly rapid and close paper. If these directions are carried out, the lead chromate will be quite granular, and will show no tendency to run through. The precipitate in the flask and on the filter is washed several times with hot water containing about 0.5 per cent, of acetic acid until free from soluble chromates. The funnel with its filter is then set over the original flask, and hot dilute hydrochloric acid (1:1) poured through the filter, dissolving the lead chromate. Further additions of hydrochloric acid are made if necessary until all lead chromate is dissolved from the filter; then it is washed with warm water until free from chromate.
The original flask now contains nothing hut the hydrochloric acid solution of the lead chromate and the washings, which, after adding a small crystal from 0.5 to 2 g. in weight of potassium iodide, is titrated direct with standard “hypo” solution whose value is known in terms of lead, the most suitable strength being that in which 1 cc. is equal to 5 mg. of lead. In this operation, by using only a small quantity of potassium iodide and having the solution fairly strong with hydrochloric acid (about 50 cc. of hydrochloric acid, 1:1, in a total of 200 cc. of solution) and somewhat warm, any tendency of the lead to form yellow scales of lead iodide, and thus somewhat obscure the end-reaction with starch, is completely checked, and the end- reaction has the same sharpness as in the back-titration of the originally described sulphate-chromate method.
This modification of the chromate method, as will be seen, does not consume, for heavy lime-ores, more than one-half the time of the sulphate-chromate method. It was so attractive that extended trials were made to see if its results in daily practice were concordant with the sulphate-chromate method which had already been thoroughly tested. Several hundred wet lead-assays were run by this method in parallel with the sulphate-chromate method not only on lime-ores, but on siliceous- ores, tailings, concentrates and artificially prepared charges, containing various additions of possibly interfering elements. It is needless to give details of all the tests. Concerning the influence of such possibly interfering elements, the results, verified by repeated trials, are as follows:
- No interference is caused by the presence of aluminum, iron, arsenic, calcium, magnesium, copper, cadmium, zinc, uranium, phosphorus, tungsten or vanadium, under the usual conditions of the assay, as previously described; the results being concordant with those of the sulphate-chromate method.
- Manganese causes no interference, but remains in solution, provided the precaution is taken to have the digestion of the ore made with sufficient hydrochloric acid in the proper ratio to nitric acid in order to insure that all of the manganese will be transformed to chloride.
- Barium, if it passed into solution, would cause high results, because barium chromate is insoluble; but this element usually occurs as sulphate and, under the conditions of the assay, it remains insoluble and is therefore unaffected by chromate solution. Even if it occurred as a carbonate, there are usually sufficient sulphates formed from the oxidation of sulphides to cause it to separate out as the sulphate salt. However, to insure this reaction, it is only necessary in dealing with ores containing barium, to add 1 or 2 cc. of a 10-per cent, solution of ammonium sulphate, along with the usual addition of ammonium-acetate. The same considerations apply to strontium salts, should they be present in the ore, with this difference, that even if some strontium did pass into solution, it would only be precipitated as chromate from a highly concentrated solution.
- Bismuth, on the addition of ammonia, forms a hydrate which, if in considerable amount, does not clarify completely with the addition of acetic acid; and, although this precipitate, on solution in hydrochloric acid, causes no reaction with potassium iodide, it is more or less bulky if present in large quantity, and it is troublesome mechanically in washing the precipitate of lead chromate. Therefore, in the presence of large amounts of bismuth, the sulphate-chromate method is the more rapid.
- Antimony forms a residue of variable composition, which, if brought on the filter along with the precipitate of lead chromate and dissolved by hydrochloric acid, reacts strongly with potassium iodide and renders the assay unreliable, so that in the presence of antimony the sulphate-chromate method, which is in this case perfectly satisfactory, must be used.
- Silver forms an insoluble silver chromate, which would be brought on the filter along with the lead chromate, and thus increase the weight of the latter by the quantity of the former present; and if this quantity is more than negligible it would entail a correction for silver, or would call for the use of the sulphate-chromate method.
As a summary of the foregoing, it may be said:
The sulphate-chromate method is applicable to the accurate determination of lead in all quantities, and in the presence of all commonly-occurring elements.
The modification of this method, by omitting sulphuric acid and bringing the ore into solution in nitro-hydrochloric acid, with the precautions already cited, is applicable, except in the presence of antimony, or in the presence of considerable amounts of bismuth or silver; and it is a decided short-cut in point of time for ores containing a high percentage of lime. For siliceous ores and products leaving so large a residue, or separation of gelatinous silica, by the initial procedure of this method as to render a preliminary filtration advisable before adding the chromate, the bulk of solution produced by this operation and its attendant washings becomes so large that, on adding the chromate solution, the lead chromate, except on standing some time, does not separate out in a form sufficiently granular to remain completely on the filter, but tends to run through; in such cases the sulphate-chromate method will be found more satisfactory and more expeditious.
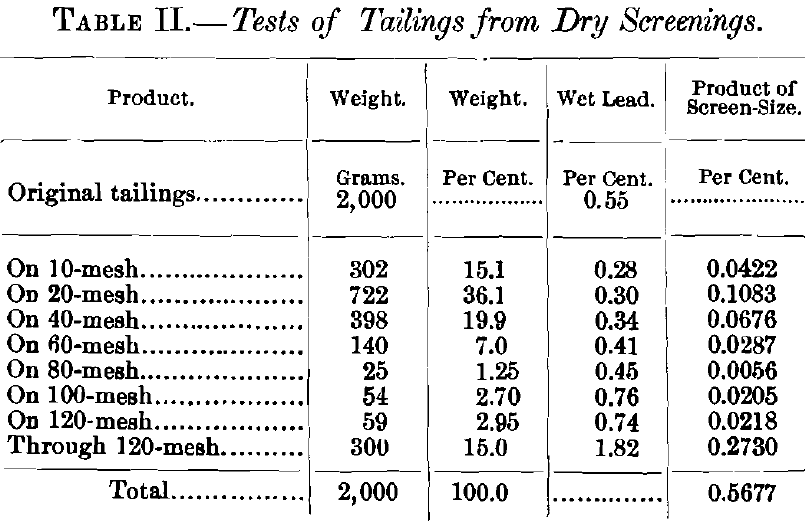
The screen-test on the tailings from a heavy lime-ore (Table II. ) gives a good idea of the class of work that can be done on small percentages by the chromate method. The lead-value, as given for each screen-size, represents in each case the mean of three determinations, and the greatest variation in any instance was less than 0.03 per cent. Two of these determinations were made by the sulphate-chromate method, and one by the modified, or short-cut, method. The assay-value of the original tailings was 0.55 per cent., and the separate assays of the screen-sizes, when afterwards multiplied by their respective weight-percentages, amounted to approximately 0.57 per cent.
A wet method capable in ordinary routine-work of yielding such a concordance in fractional lead-percentages is, it would seem, worthy of consideration on the part of analysts.
Postscript.—Since working out the above sulphate-chromate method and the modified, or short-cut, method, I have learned that, although never introduced to any extent into commercial practice, a method somewhat analogous to the former was devised some years ago, and, although my own work was quite independent, I wish all merit of originality to be placed with the earlier investigators where it belongs. So far as I am aware, however, the modified or short method, which is particularly applicable to low-grade, heavy lime-ores,for which the molybdate method is especially weak, has never been previously described.
The Commercial Wet Lead-Assay. BY H. A. GUESS
