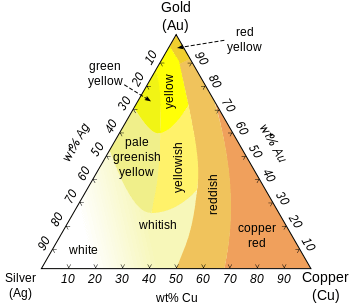
Gold often comes as an alloy and for that will be of various color. Gold colouring goes from that bright yellow we all know to White, Rose, Pink, Red Gold, Green, Blue, Purple and Black Gold.
Depending on the allow mix, you can derive or metallurgically fabricate colors.
illustrates it well.
- White Gold: For gold to take a white color, it must be mixed with a white metal such as nickel, manganese or palladium. Standard White gold is usually 14K of gold (58.5% purity) while the rest is divided as 21% copper, 7.84% zinc, and 12.73% nickel. and White gold can often be rhodium plated to give it a more shiny and white appearance.
- Rose, Pink, Red Gold: Gold can take these colors when mixed with copper. The more copper in the alloy, the darker the tone of red that will surface. A common rose gold alloy composition is 18K (75% gold) mixed with 25% copper while a 50/50 mix of gold (12K) with copper results in what we would call red gold.
- Green Gold: Green gold, otherwise known as electrum, is a natural forming alloy which combines gold and silver. The greenish color varies depending on the exact mixture but back in the 73% gold, 27% silver
- Blue Gold: 46% gold, 54% indium.
- Purple Gold: 80% gold, 20% aluminium.
- Black Gold: 75% gold, 25% cobalt.
https://www.webexhibits.org/causesofcolor/9.html complements it well in talking about gold alloys in saying that metals are coloured because the absorption and re-emission of light are dependent on wavelength. Gold and copper have low reflectivity at short wavelengths, and yellow and red are preferentially reflected, as the colour here suggests. Silver has good reflectivity that does not vary with wavelength, and therefore appears very close to white.
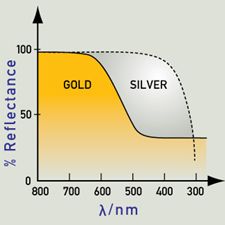
When two metals are dissolved in each other (as is the case with alloys), the colour is often a mixture of the two. For example, copper dissolved in gold changes the color from a yellow-gold to a red-gold. Silver dissolved in gold creates a green-gold color. White gold contains palladium and silver. The colour of gold jewellery can be attributed to the addition of different amounts of several metals (such as copper, silver, zinc, and so on).
The lustre and fine colour of gold have given rise to most of the words which are used to denote it in different languages. The word “ gold ” is probably connected with the Sanscrit word “ jvalita,” which is derived from the verb “jval to shine. It is the only metal which has a yellow colour when in mass and in a state of purity. Impurities greatly modify this colour, small quantities of silver lowering the tint, while copper raises it. In a finely divided state, when prepared by volatilization or precipitation, gold assumes various colours, such as deep violet, ruby and reddish-purple, the tint varying to brownish- purple and thence to dark brown and black. This purple colour has been supposed by some experimenters (viz., Guyton de Morveau, Buchner, Desmarest, Creuzbourg and Berzelius) to be due to the formation of a coloured oxide of gold of unknown composition, but Buisson, Proust, Figuier and, more recently, Kriiss have shown that no oxygen can be obtained from this coloured material, and that it probably consists of metallic gold. Similar colours are seen in purple of Cassius, and in Roberts-Austen’s purple alloy of aluminium and gold, the colour in each case being probably due to a particular form of finely divided gold. The ruby coloured gold, suspended in a liquid in which it has been precipitated by ether and phosphorus, is present in the most finely divided state obtainable, and perhaps it then approaches the condition of separate atoms. Such gold shows no tendency to settle by gravity even after being kept undisturbed for several years. Somewhat less finely divided gold gives a faint blue tinge to light transmitted through the liquid in which it is suspended. Gold precipitated from its solution as bromide is in a different molecular condition from that formed from chloride, giving out 3 ‘2 calories in passing into the latter state. The surface colour of small particles of native gold is often apparently reddened by being coated with translucent films of oxides of iron. Very thin plates of gold are translucent, and appear green by transmitted light, while remaining yellow by reflected light. On heating, the green colour changes to ruby red, but is restored by the pressure of a hard substance by which the state of aggregation is again altered (Faraday). Molten gold is green, and its vapour is also probably greenish.