The Merrill System or method or Zinc Dust Precipitation was introduced in 1897 at Marysville, Montana; in the plant of the Montana Mining Co., and subsequently at the Homestake Mine in South Dakota, where 130,000 tons of ore are treated per month and 4000 tons of solution precipitated every 24 hours.

With very few exceptions this process has been installed in all of the large mills built during the last two years in the United States, Mexico and Canada, and has recently been introduced in South Africa, where 5 installations are now (1913) in operation.
The general arrangement of the plant is shown in Figs. 37a and 37b.
The rich solution to be precipitated is collected in one or more sumps. A pump, preferably of the plunger type, is placed with its suction connected to this rich solution sump and its discharge leading to a filter press, usually at a higher level.
The zinc dust is mixed with the solution at a point in the suction line about midway between the pump and the tank.
The apparatus for controlling the supply of zinc consists of a hopper suitably mounted and provided with a worm discharge. The dry zinc powder from this feeder is mixed and ground with solution in a small tube-mill or mixer and then flows directly to the suction of the pump. Both feeder and tube-mill are operated
from a line shaft or small motor and, by means of an adjustable friction drive on the worm, the discharge of zinc may be regulated to suit the flow of solution which is drawn from the sump and forced through the filter-press.
The frames of the Merrill press are triangular in section (see Fig. 37c), and the feed pipes are so arranged that the solution and zinc enter from the bottom or apex of each compartment. In consequence of this form the precipitate as it accumulates in the frames is kept constantly agitated and the full precipitating efficiency of the zinc is obtained.
The clear barren solution leaving the filter press flows to a storage tank and may be used as required in the mill.
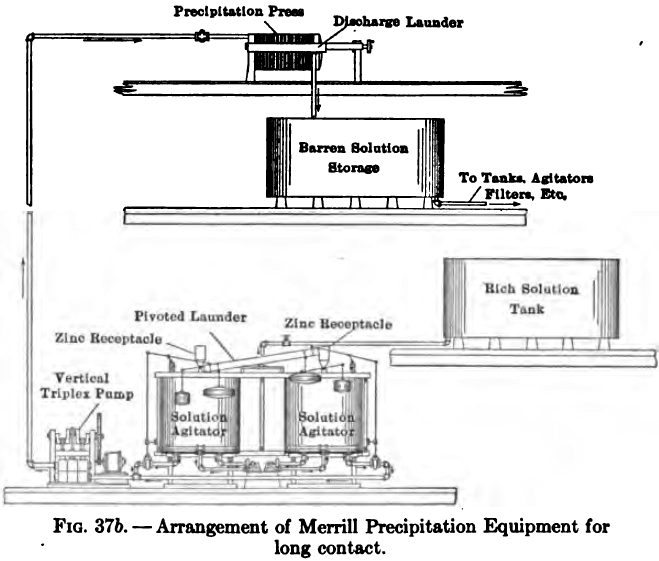
In cases where a long contact between zinc and solution is required, as in solutions weak in cyanide and low in gold, the arrangement shown in Fig. 37b is used.
The rich solution is collected in a main sump or storage tank and from this flows alternately to one of two smaller tanks. Each of these small tanks is connected to the suction of a pump and in turn to a filter-press, as before described. By means of an arrangement of floats and levers, the filling and emptying of these small tanks from the main sump is entirely automatic, one being pumped through the filter-press, while the other is filling. By means of two additional floats a zinc-dust feeder is made to discharge into each tank, every time it is filled, a given amount of zinc.

Each of the small tanks contains an agitator by means of which the mixture of zinc or precipitate and solution is taken from a point at the bottom of the tank and circulated by means of a centrifugal pump through revolving arms provided with a series of small nozzles. In this way the contents are thoroughly mixed, and by regulating the time at which the floats operate, any desired length of contact between zinc and solution may be obtained.
This system of using two tanks alternately provides an accurate measure of the tonnage of solution precipitated and thus furnishes a check on the actual bullion recovery, which should correspond with the difference between head and tail assays of the solution.
Advantages of the System.— The principal advantages claimed for the zinc-dust precipitation process are:
(1) More uniform and efficient precipitation, owing to the extremely fine state of division and consequent large precipitating surface exposed. Since the filter-cloths become coated with a layer of fine zinc, the whole of the solution comes in intimate contact with the precipitant. Hence a very short time of contact is necessary for effective precipitation.
(2) Lower consumption of zinc. Owing to the short time of contact, but little zinc is dissolved beyond that required for actual precipitation.
(3) Cost of zinc-dust is 20 to 25 per cent, less than that of shavings.
(4) Less labor and handling of precipitate. The clean-up with zinc dust is much simpler and quicker than with shavings. The presses are dried with air to about 30 per cent, moisture, then opened and the cakes of precipitate dumped into cars, which are sampled and weighed to determine the actual dry weight of precipitate.
(5) Purer product for smelting. In cyaniding silver ores with zinc-dust the precipitate obtained may contain from 75 to 85 per cent, of pure silver, which may be fluxed and melted without preliminary treatment.
(6) Complete clean-up possible. With shavings precipitation a large part, say 25 to 40 per cent., of the total precipitated values are returned to the boxes after a clean-up, along with the shavings not consumed. With zinc-dust all the values are converted into bullion.
(7) Less danger of theft, since the precipitate is locked in the heavy cast-iron filter-press until this is opened for the clean-up. Moreover, the uniform ratio of zinc-dust to bullion serves as an additional check on theft.
(8) Special methods may be used to increase extractive power of solutions. When sodium sulphide, for example, is added for this purpose, it causes a boiling over in ordinary zinc shaving boxes, owing to violent action of the alkali on the zinc. With the system of agitation and filter-pressing this trouble does not occur.
The chief objection to the zinc-dust method of precipitation is that it requires more skill in operating than the shavings method. Formerly it was supposed that the consumption of zinc would be greater and the grade of bullion lower with dust than with shavings, but under proper conditions the contrary is found to be the case.
Comparative Results with Dust and Shavings
The following figures were obtained at the plant of the Homestake Mining Co., South Dakota (A. J. Clark, Journ. Chem. Met. and Min. Soc. of South Africa, Jan. 1909), the comparison being between 12 months’ use of zinc shavings in 1906 and 9 months’ use of zinc-dust in 1908. It is to be noted that solutions below .03 per cent. KCN could not be satisfactorily precipitated by shavings, whereas solutions as low as .015 per cent, were successfully handled with zinc-dust.
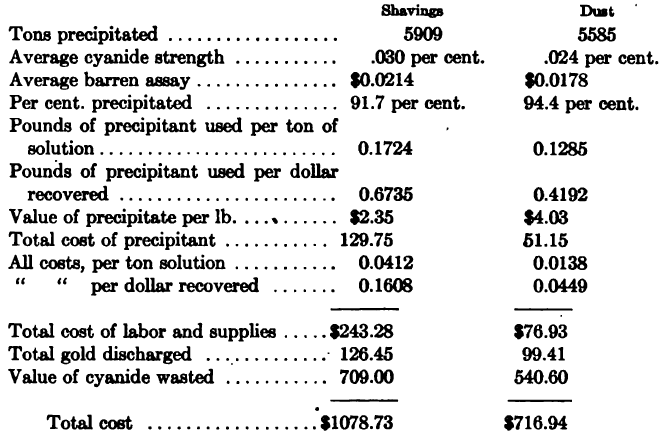
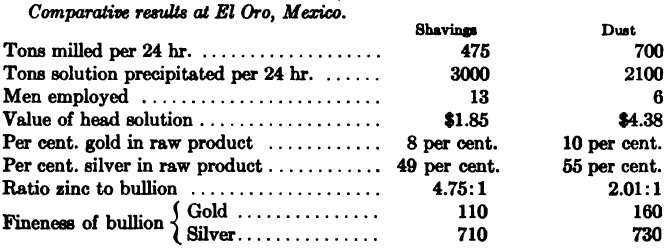
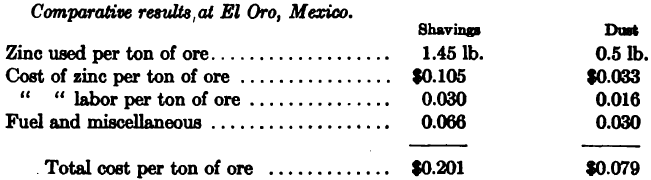
The following was the consumption of zinc-dust at various plants using the process described above: