Part of Zinc Hydrometallurgy and Zinc Solvent Extraction includes the zinc and iron sulphate solution from the hot acid leach will typically contain 80-100 g/l Zn, 20-30 g/l Fe, mostly in the ferric state, and 40-60 g/l free sulphuric acid. The sulphuric acid concentrations will depend upon the leaching techniques used, and the composition of the zinc calcine.
In the iron extraction process the sulphuric acid has to be neutralized either by zinc- Versatic in the extraction stages or by calcine in a separate preneutralization step. If the sulphuric acid concentrations are moderate, say up to 40 g/l, the hot acid leach solution may preferably be contacted directly with the zinc loaded organic phase. The sulphuric acid will then be neutralized as described by Equation (4) prior to the exchange reaction, Equation (3).
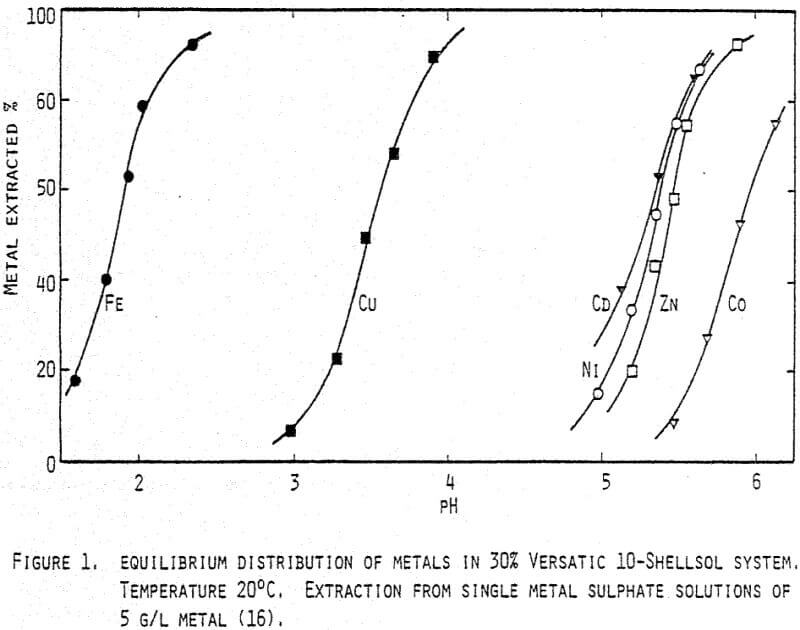
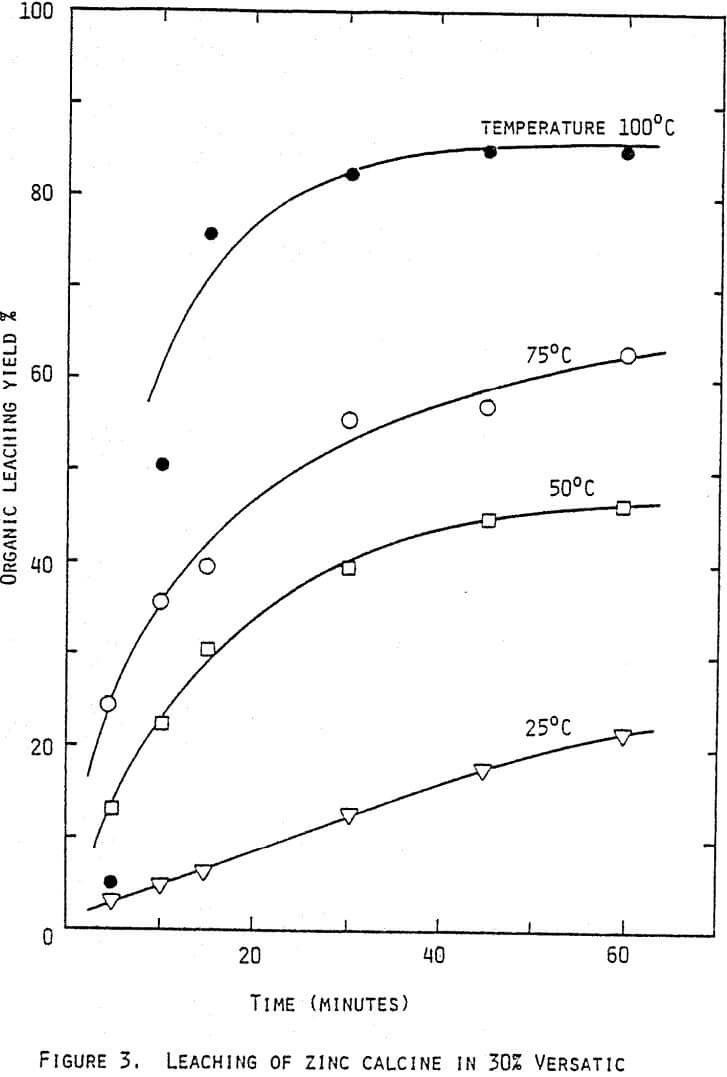
A typical calcine contains about 52% zinc and 8% iron, giving a zinc to iron ratio of 6.5. Extracting 1 kilogramme of ferric iron will need an equivalence of 1.75 kilogramme of zinc in the form of Zn-Versatic in the organic phase. When leaching zinc calcine in a 30% Versatic solution, a typical leaching yield of zinc may be 70%.
Thus the feed in the organic leach has to contain 2.5 kg zinc (4.8 kg calcine) for each kilogramme iron to be extracted. If the zinc to iron ratio in the calcine is 6.5 it follows that about 40% of the total calcine is to be applied in the organic leach to produce enough zinc-Versatic for the iron extraction.
With the same calculations for sulphuric acid it is found that 0.95 kg zinc (1.8 kg calcine) will be needed as feed flow to the organic leach for neutralizing one kilogramme sulphuric acid in the aqueous phase from the hot acid leach. If the aqueous phase contains 20 g/l iron and 40 g/l sulphuric acid, the total amount of zinc entering the organic leach will be 4.4 kg zinc (8.4 kg calcine) for each kilogramme of iron extracted. An overall balance shows then that about 67% of the calcine will be needed in the organic leach.
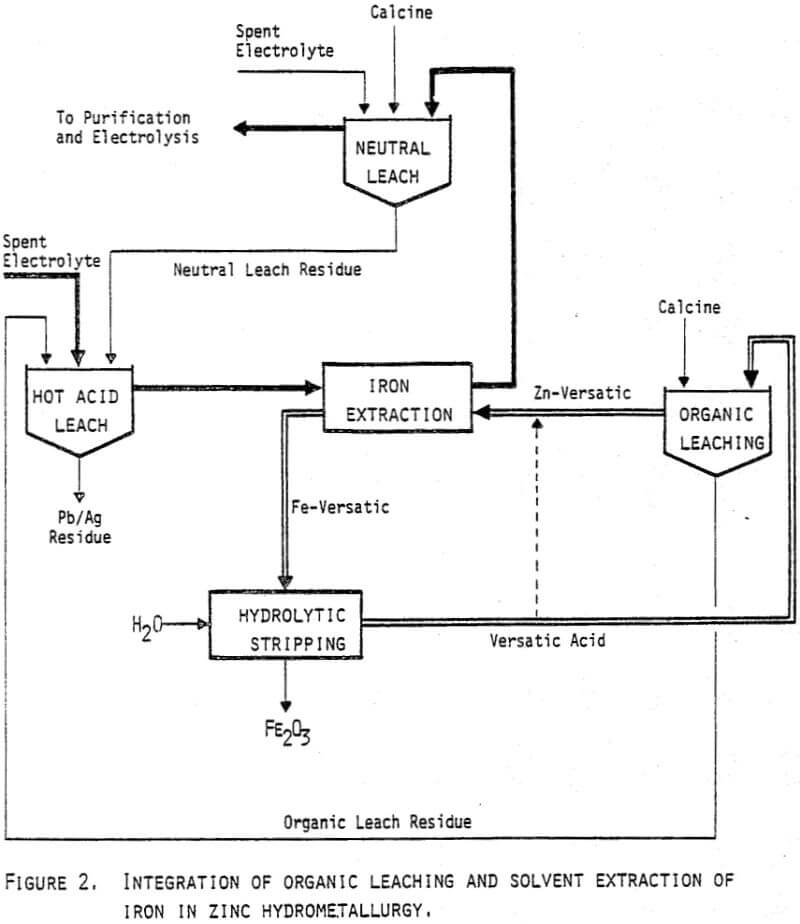
From the above example it has been shown that only one third of the calcine is fed into the neutral acid leach (Figure 2). Obviously a number of balances and modifications can be made. If the final concentration of free acid in the hot acid leach solution is rised, some of the calcine may be used directly in a separate stage for preneutralization prior to the solvent extraction circuit.
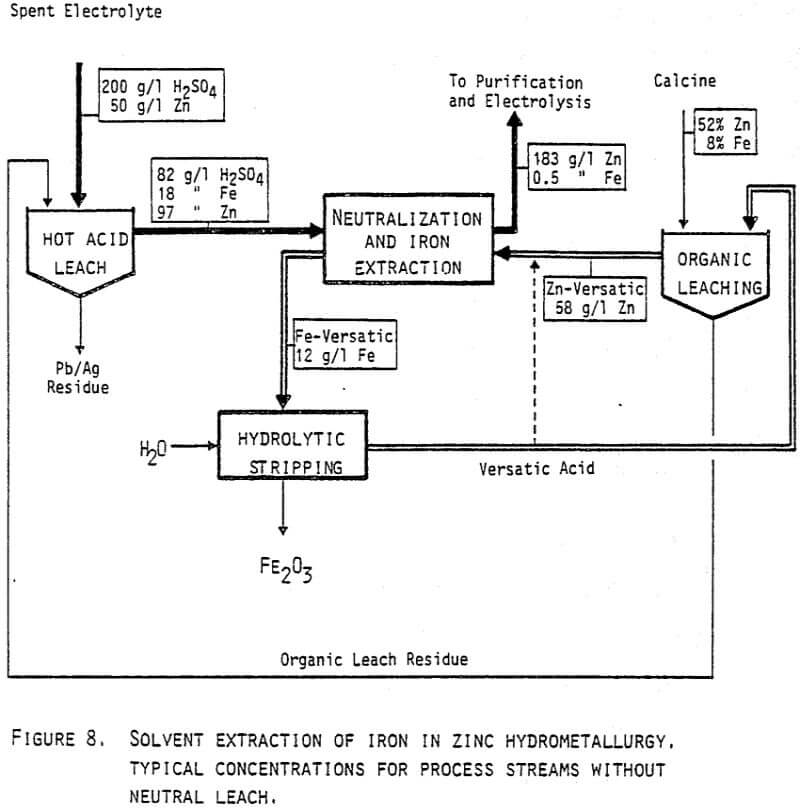
The most radical change from present practice in zinc hydrometallurgy would be to completely omit the neutral acid leach. This situation is shown on the flowsheet in Figure 8. With the same overall zinc to iron ratio (6.5) and with 60% yield in the organic leaching of zinc, the circuits will be in balance by the typical flows given on the figure.
Zinc Hydrometallurgy
The use of solvent extraction techniques for removal of iron in zinc hydrometallurgy, as presented in this paper, may give rise to a number of questions which have not been discussed. In this paper basically the relation between iron and zinc has been treated. However, there are obviously interesting possibilities for using solvent extraction for other important elements in the zinc leach liquors, for example copper and cadmium. Current work is in progress in this area.
Introduction of solvent extraction of iron may quite clearly have an effect on impurity levels. However, a third generation zinc plants will be based upon the well establised technique of hot acid leach as well as the further purification steps such as precipitation of a minor amount of iron as ferric hydroxide for scavenging impurities such as As, Ge and Sb, and the purification step with zinc dust cementation.
Thus it is believed that the present generation of zinc plants can readily be adjusted to incorporate solvent extraction of iron replacing the precipitation of jarosites and goethite. The advantages to be gained may be summarized as:
- increased zinc recovery,
- making the iron as a product of commercial value, probably as a feed material for iron works, thus at least saving deposit cost,
- because of the inherent possibilities within the solvent extraction technique itself, the iron oxide product may meet various specifications to make it suitable for pigments, magnetic ferrites, iron powders etc.,
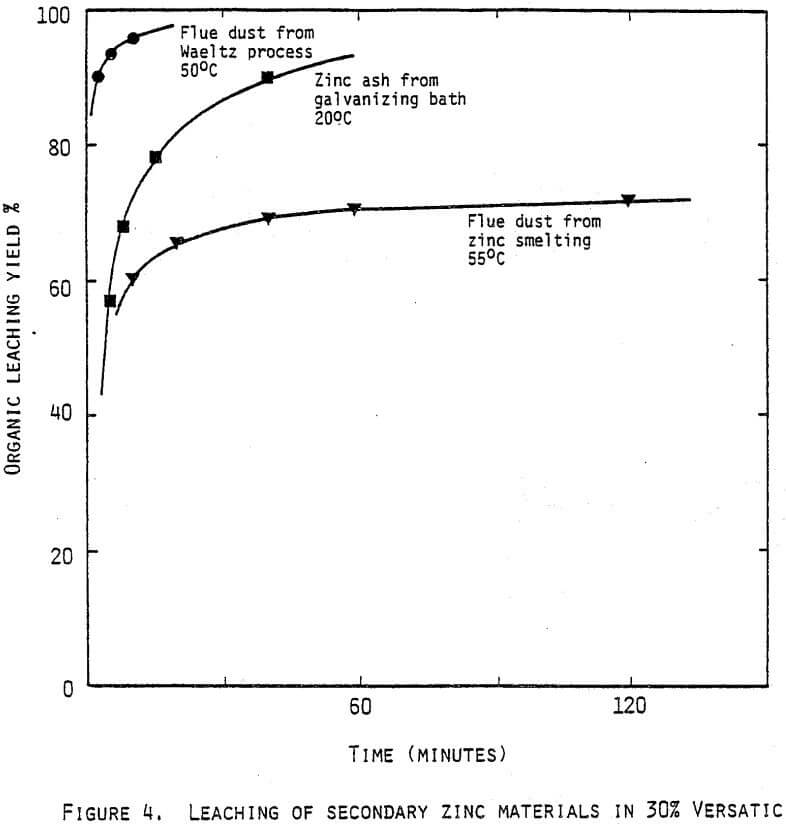
- more use can be made of zinc-containing scrap materials such as fume oxides, flue dusts, galvanisers drosses; these materials are excellent sources for organic leaching,
- zinc plant residues may be reduced to a “pure” hot acid leach residue; in most cases an improved Pb/Ag-residue will appear, more attractive for further upgrading than residues mixed with jarosites, goethite or zinc ferrites.
Since the introduction of the electrolytic process for the primary production of zinc, which has been seen in commercial operation for more than sixty years, iron has played, and indeed still plays a particular and very significant role in the technology and economics of the process.
The main raw material in zinc production is zinc sulphide concentrate with about 50-55% Zn. These concentrates will always contain a certain amount of iron, typically in the order of 5-12% Fe, both in the form of pyrite (FeS2) and as the mineral marmatite where iron replaces some of the zinc in the sulphide matrix. In the roasting process, which converts the sulphides to oxides, most of the present iron combines with zinc forming zinc ferrites. These are complex oxides represented by the formula ZnFe2O4 and may be considered as a key component in the historical development of zinc hydrometallurgy.
The pure zinc oxide part of the roaster calcine is readily dissolved in the dilute sulphuric acid while the zinc ferrites may be left as a residue virtually insoluble by adjusting the conditions to a suitable temperature and acidity in this operation. During the first generation hydrometallurgical zinc plants, the recovery of zinc was deliberately limited to the dissolution of the zinc oxide by careful leaching with spent electrolyte containing about 50 g/l Zn, and 150-200 g/l H2SO4. The zinc ferrites were not dissolved and in this way iron was kept at a very low level in the resulting zinc sulphate solution.
On the other hand, this low level of iron, say 0.5-1.0 g/l, was of advantage in that by neutralization to pH 4-5, iron was precipitated as ferric hydroxide which has a purification effect by scavenging small amounts of impurities such as As, Sb and Ge. These elements have to be removed to levels of one part per million or less in order to make the electrowinning process operable .
Although it was well known that the zinc ferrites could be broken down by the attack of hot sulphuric acid, the dissolution of the ferritic zinc would be accompanied by the simultaneous dissolution of the iron in the ferrites. This will introduce an iron concentration at the level of 10-25 g/l Fe in the leach solution, which was far too high to be treated by conventional ferric hydroxide precipitation or any technically feasible process known at that time.
Thus in the first generation of hydrometallurgical zinc plants the deliberately limited dissolution of zinc from the roaster calcine resulted in overall zinc recoveries of only 85 to 93%. Depending on economic considerations a further recovery of the remaining zinc had to be taken care of by additional pyrometallurgical processes such as a sulphation roast, slag fuming or Waeltz processes.
A second generation electrolytic zinc plants was seen by a particular breakthrough in the hydrometallurgical processing of the zinc ferrites in the residue. The ferrites were allowed to be dissolved in hot sulphuric acid when it was shown that large quantities of iron could be precipitated as basic iron sulphates, known as jarosites, from acid solutions at about pH 1.5. The jarosites may be presented as MFe3(SO4)2(OH)6 where M is a monovalent cation such as Na+, K+ or NH4+. The jarosite process has been presented and discussed in various versions by Steintveit and Gordon and Pickering.
The technological breakthrough was achieved by the formation of the jarosites as a crystalline, easily filterable material, which could be readily separated from the zinc sulphate solution obtained from dissolving zinc ferrite in hot sulphuric acid (spent electrolyte). Thus the overall recovery of zinc was no longer limited by the leaching operation. In most of the modern zinc plants more than 99% of the present zinc is brought (or may be brought) into solution by introducing a hot acid leach (HAL) for dissolving the zinc ferrites in addition to the first step of a neutral leach of the pure zinc oxides of the calcine.
In addition to the Jarosite process, the last decade has also seen the development of other methods of precipitation of iron in a crystalline form such as the Goethite process (FeOOH) and the Hematite process (Fe2O3). An extensive review and discussion of the advantages and disadvantages of the various processes and their modifications has been presented by Monhemius.
By the improved leaching and precipitation techniques introduced in the second generation zinc plants, the overall recovery of zinc have risen to 95-97%. Although above 99% of the zinc may be brought into solution by the improved leaching techniques, there is a discrepancy between this figure and the actual overall recovery. This is mainly due to the fact that the precipitation operations produce final residues from the zinc plants where some zinc is lost.