Table of Contents
Greenockite is the only known cadmium mineral of importance. It occurs rather universally, in minor concentrations, as a secondary mineral in sphalerite deposits. The world’s cadmium output is obtained through the processing of metallurgical by-products, largely from the treatment of residues from electrolytic zinc, retort zinc and lithopone plants. These sources are supplemented by the processing of fumes from lead and copper smelting operations. The development of modern selective flotation practice in the decade 1920-1930, which permitted the economical mining of complex lead- zinc ores, resulted in significant increases in the quantities of cadmium entering lead smelting systems.
Preparatory Processing
The cadmium content of lead smelter receipts is low and, as a rule, proportionate to the zinc content; the usual range of cadmium contained being of the order of 0.01-0.05 pct. Were it not for the low boiling point of cadmium, such small concentrations would, no doubt, be lost in the large tonnages of slag, metal and other smelter end products. However, the ready volatility of cadmium and its compounds at prevailing lead smelting temperatures results in its concentration in fractional portions of fume collected. This collected fume comprises a circulating load within the smelter system.
Thus, the cadmium content of blast furnace fume increases with each successive circulation until an equilibrium value is reached when the sum of the cadmium losses, due to handling and in slag, waste gases and other end products, becomes equal to the intake as ore. With ore receipts averaging, say 0.03 pct cadmium, the concentration value obtainable, through fume circulation in a routine manner, approaches 10-12 pct. In such operations, cadmium concentrations in blast furnace fume of from 3-5 pct are readily attainable. However, the concentration gain beyond this range, with each additional circulation, is progressively decreased as a result of mounting losses occurring through handling and in end products.
In the fusion process, as in the more common method described above, blast furnace fume is circulated in the regular lead smelting system until a cadmium concentration of 4-6 pct is reached. It is then removed, mixed with a siliceous flux and fused in a special reverberatory operation. During the fusion, the bulk of the cadmium is distilled and the bulk of the lead retained as a silicate slag. Lead silicate slag is tapped and returned to the blast furnace; the cadmium fume is bag filtered, then sacked for delivery to a cadmium leach plant. In reverberatory melting, the grade of cadmium fume produced is limited by the necessity of excessive temperature in the upper bath layer.
Port Pirie Works—Broken Hill Associated Pty., Ltd., Port Pirie, Australia
Blast furnace fume from routine lead smelting operations is continuously advanced to a central sump and agitated with water for the solution of fractional portions of the cadmium present. The pulp is then subjected to vacuum filtration, giving two products: (1) Residue, which is returned to the blast furnace and (2) Filtrate, which is treated with sodium carbonate for the precipitation of dissolved cadmium. The cadmium carbonate precipitate is filtered, dried and shipped to the Electrolytic Zinc Co. of Australasia, Ltd., at Risdon.
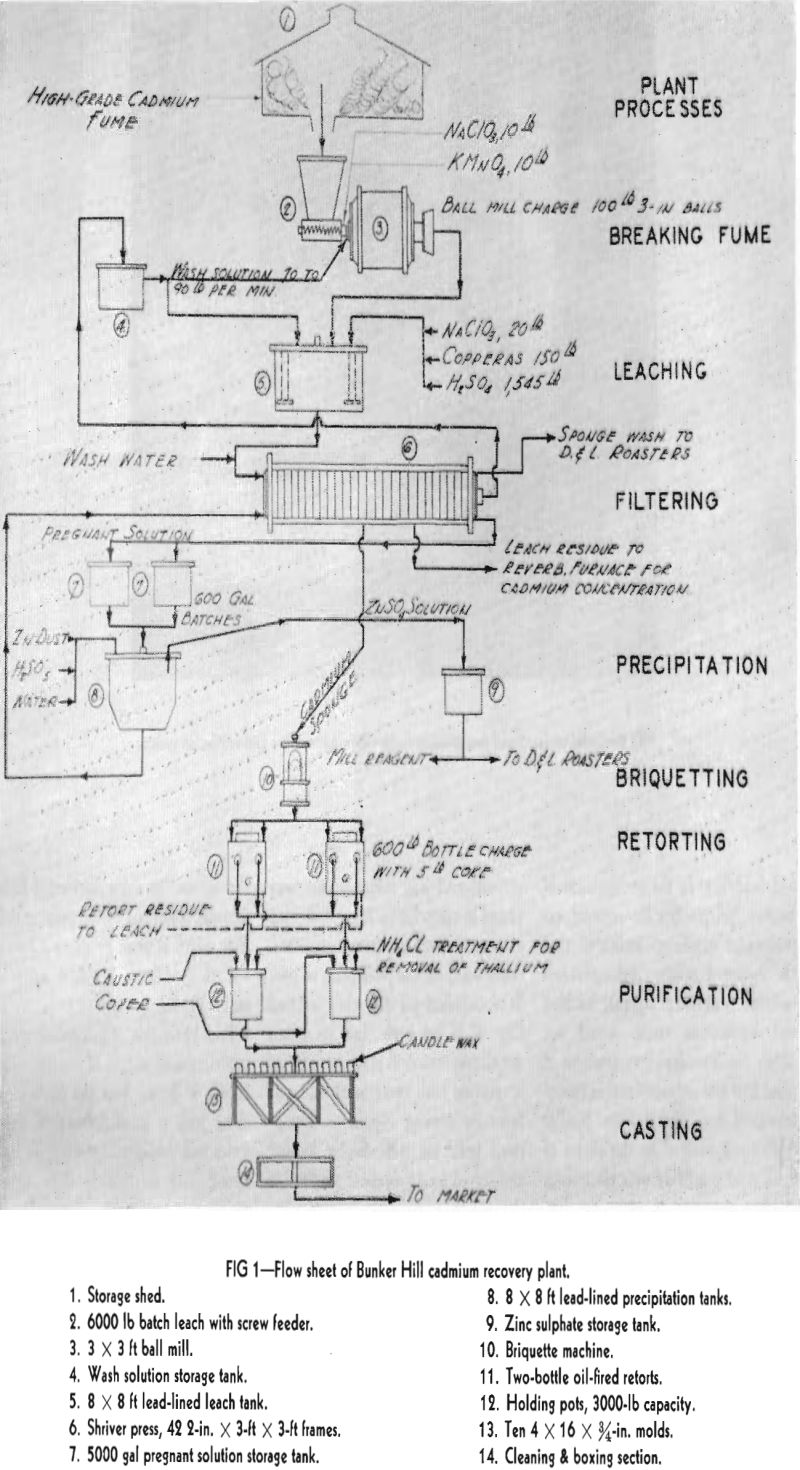
Leaching and Purification Practice
Referring to the accompanying flowsheet, sacked cadmium fume is weighed and dumped into a steel bin which is screw-discharged into a 3 x 3 ft pulping ball mill located on the leach floor. Circulating wash solution meets and carries the screw-discharged fume into the scoop box of the pulping mill from which it flows by gravity into a vented, lead-lined, 8 x 8 ft agitator tank.
Sponge Precipitation Practice
After two leach days, during which the solution of 8000 to 10,000 lb of cadmium is effected, the crew turns to precipitation operations. Stored pregnant solution, now of known cadmium content, is drawn in 600-800 gal batches into a lead-lined, conical-bottomed precipitation tank served by a high-speed agitator. As in the leach system, this tank is hooded and positively vented by means of a 6400 cfm fan. The vented tank atmosphere is conveyed and discharged into the blast furnace flue system. In this manner, possible hazardous concentrations of arsine are speedily removed and diluted to harmless proportions with 150,000 cfm of blast furnace gas.
Metal Recovery Operations
Sponge briquetting, with no additive agents and no prior treatment other than granulation through an 8-mesh screen, is practiced. The screened sponge is charged to the hopper of a Model R Stokes machine. The sponge is dewatered and its density increased about three times, by compression under 17 tons pressure, into 2 in. diam by ½ in. thick briquettes. The compressed discs discharge into steel storage pans at the rate of about 1000 lb per hr. Each storage pan has a capacity equivalent to that of a retort bottle. They stand off the floor on legs to permit ready transfer to the retorts by lift truck.
Two oil fired retort units are employed for cadmium distillation from briquetted sponge. Each retort unit is of firebrick construction and contains two No. 11 American Crucible Co. graphite bottles set on piers above a brick checker-work arch, which dis¬tributes the heat from the firebox below. The retort bottles are manually charged, 600 lb per bottle, 1200 lb per retort unit.
Refining and Casting Practice
Two 3000-lb capacity, electrically heated kettles are used for both refining and casting. One pot is being either held or used for casting while the other is being charged for refining. Refining operations are chiefly concerned with thallium removal. The crude retort metal produced meets all impurity specifications except for lead and thallium. All metal is treated for thallium removal; occasional lots destined for sale to bearing manufacturers must be redistilled to meet lead specifications.

