Why is cyanide heap leaching used to extract gold from some deposits
Cyanide is used in heap leaching to extract gold from some low-grade deposits because of its ultra-low-cost and high effectivity are leaching gold.
How to Separate of Tantalum from Columbium
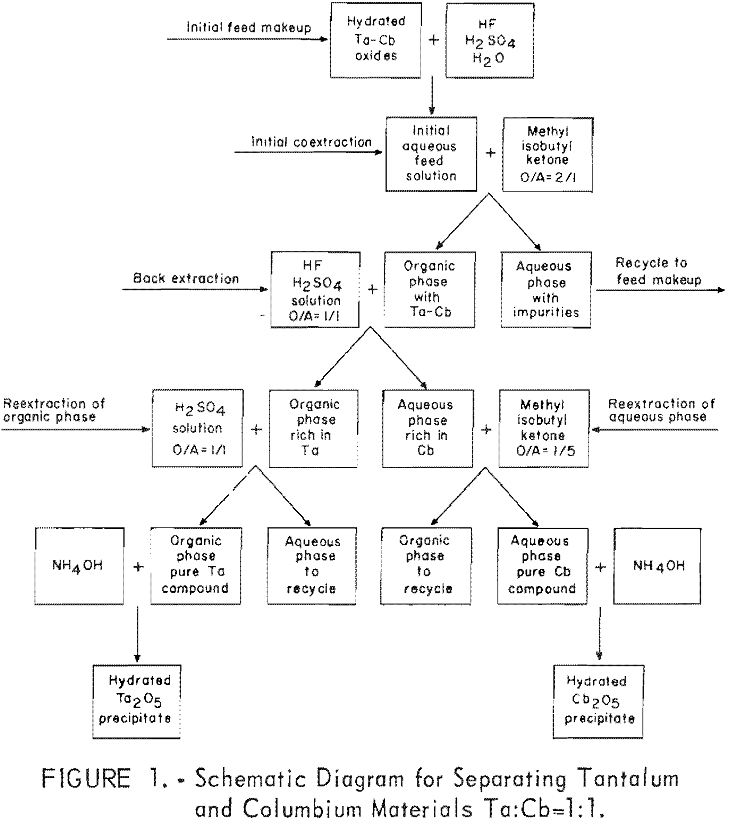
Results of this investigation demonstrate that high-purity columbium and tantalum compounds can be obtained by application of the solvent extraction separation system: Hydrofluoric acid-sulfuric acid-methyl isobutyl ketone. This system is unique in that it not only separates columbium from tantalum, but separates these two metals from other metallic impurities. The system is effective for separating […]
How Susceptible are Organic Compounds to Tritium Exchange Labeling
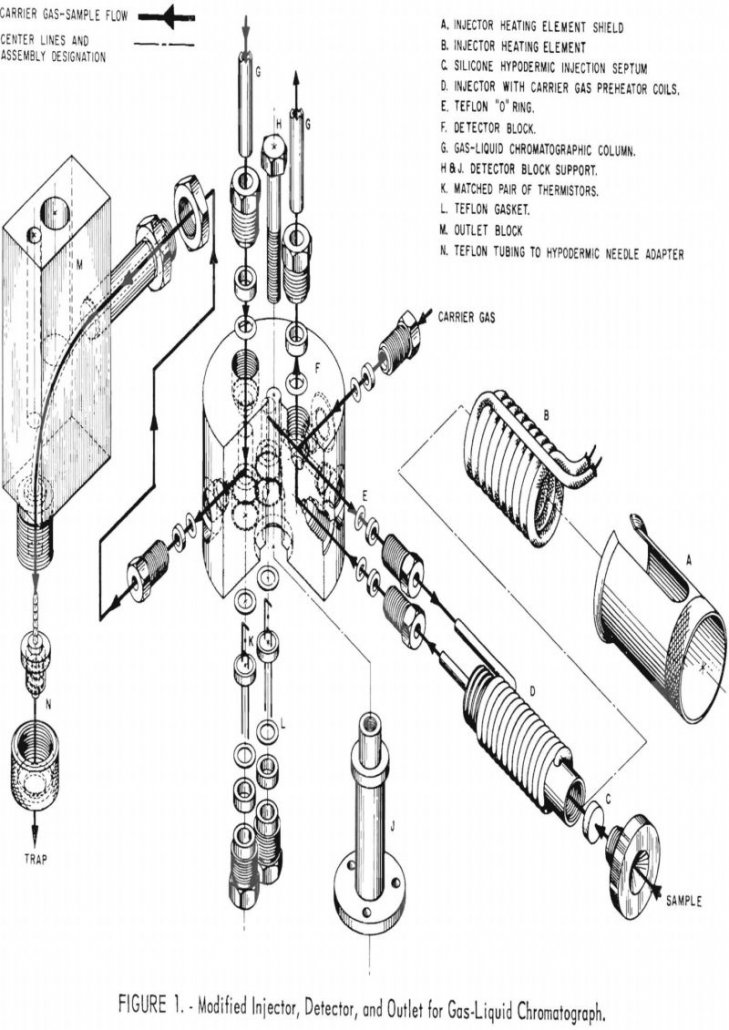
This report describes the preparation of tritium-labeled organic compounds to be used by the Federal Bureau of Mines in studying the role of certain gasoline constituents in gum-forming mechanisms. The gasoline constituents that have been investigated boil within a range of approximately 90 to 230° C. and can be classified as paraffins, naphthenes, olefins, diolefins, […]
Bacterial Copper Leaching

Among several suggested new processes for treating marginal ores in the United States, none is more intriguing than the possibility of extracting metals from low-grade materials by the action of microorganisms. One phase of a Federal Bureau of Mines investigation toward developing such a procedure was to isolate and identify specific chemosynthetic iron- and sulfur-oxidizing […]
Volatilization of Tin Chlorides

More than 95 percent of the total tin in Bolivian mine-run ores can be volatilized and recovered as tin chlorides at temperatures ranging from 520° to 565° C. Equally high percentage recoveries of tin can be obtained from low-grade Bolivian concentrates at temperatures of 565° to 600° C. Minus 35-mesh ores and concentrates can be […]
Extraction of Rare Earth Elements
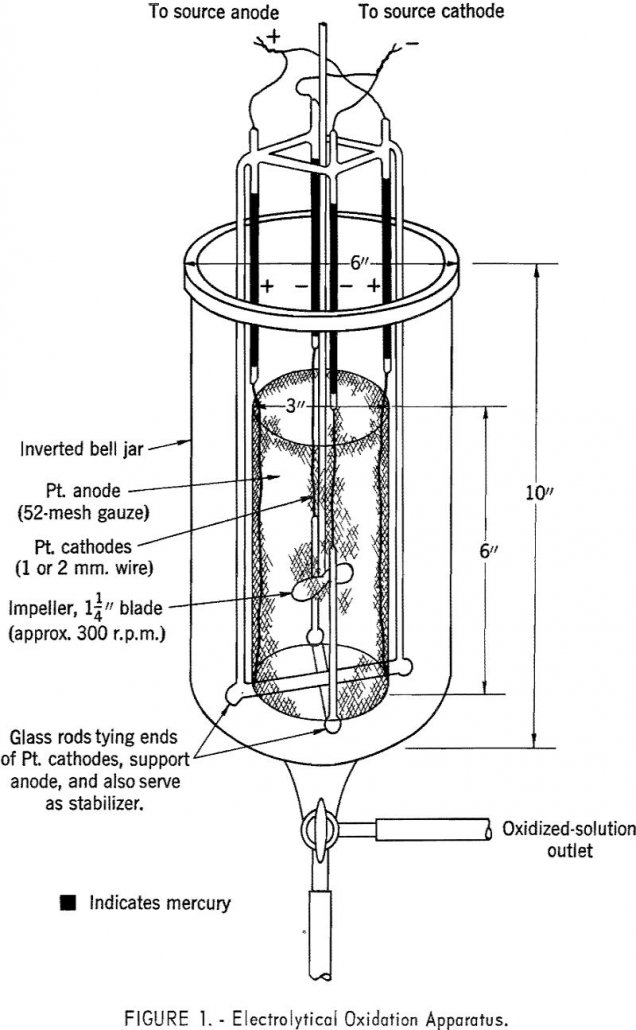
In separating and purifying rare-earth compounds, ion-exchange and liquid-liquid extraction techniques have exhibited the greatest potential. Of the two, ion exchange has received major attention and has been exploited into commercial operation. Notable research has been reported on solvent extraction techniques, especially in extraction of tetravalent cerium with phosphates and phosphonates. Work along this line […]
How to Prepare High Purity Yttrium

This report does not constitute a finished investigation. Its purpose is to report on the status of current reduction studies and to demonstrate that high-purity ductile yttrium metal can be prepared by metallic reduction of yttrium chloride. Although both lithium and sodium have proved to be effective reductants, no conclusions can be reached on which […]
Nitric Acid Oxidation Rates of Coal
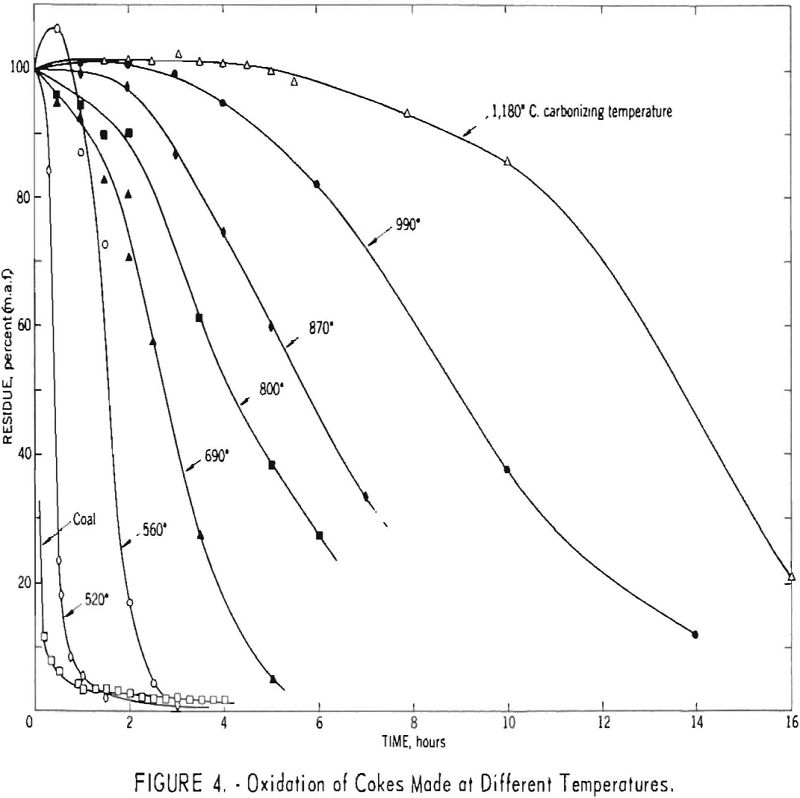
Coals ranging in rank from lignite to anthracite, cokes carbonized at temperatures ranging from 520° to 1,180° C., and synthetic polymers have been oxidized by the standard method, that is, by boiling in 8-normal nitric acid. Some of these materials were also oxidized by one or two modifications of the standard method. Although the fusain […]
How to Process Gold Ores by Heap Leaching & Carbon Adsorption Methods
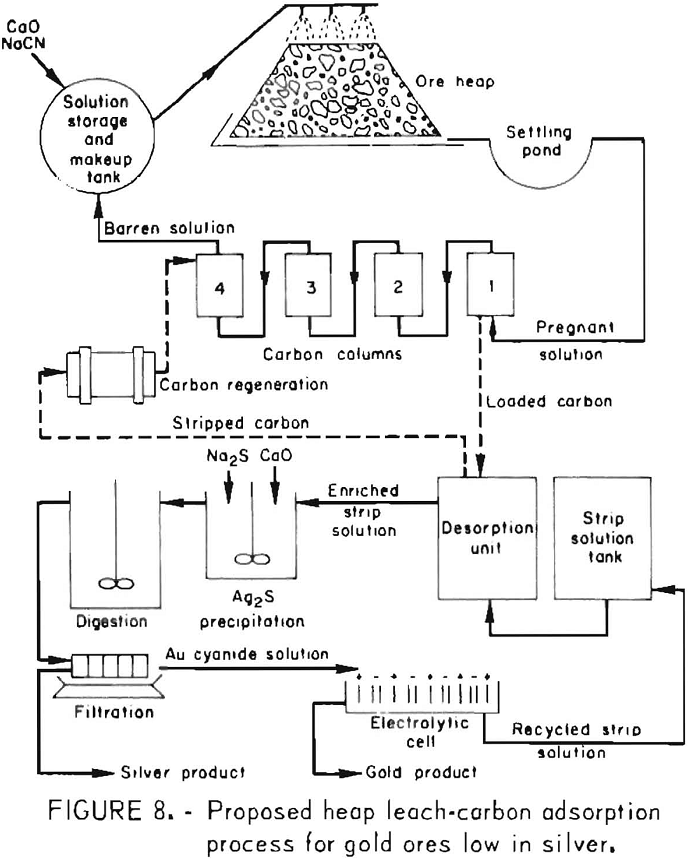
The heap leach cyanidation carbon adsorption electrowinning process developed has proved to be an economical method for exploiting low-grade gold ores and small isolated deposits not suitable for treatment by conventional cyanidation procedures. Heap leaching may be the most profitable method for processing selected gold and silver ores that do not require fine grinding and […]
Vacuum Distillation – Remove Volatile Metals
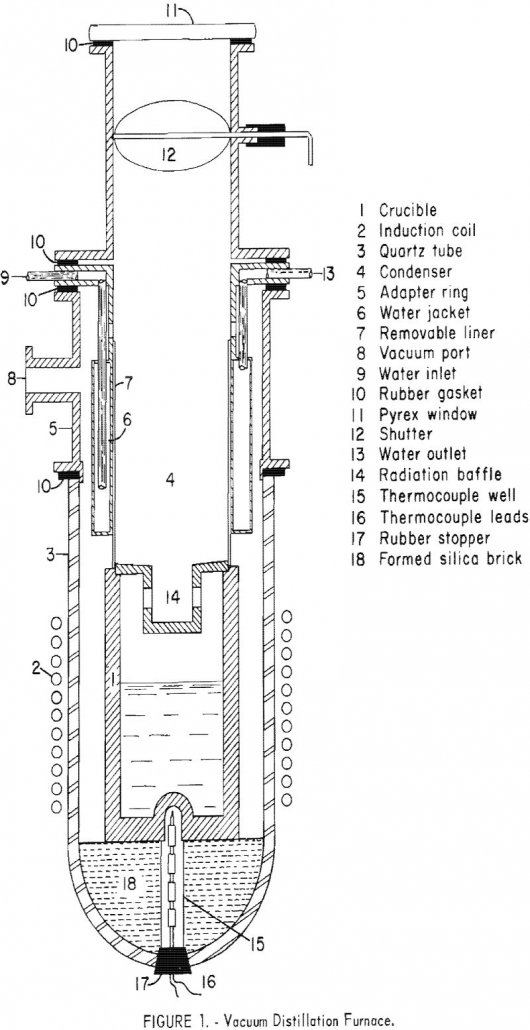
The rate of evaporation of a volatile metal from a metallic solution containing less volatile metals is determined by its partial pressure and its diffusivity. The concentration of a volatile metal will be less at the surface than in the interior of the metal; it will adjust itself to keep the rate of diffusion and […]
