Hot Potassium Carbonate Absorption
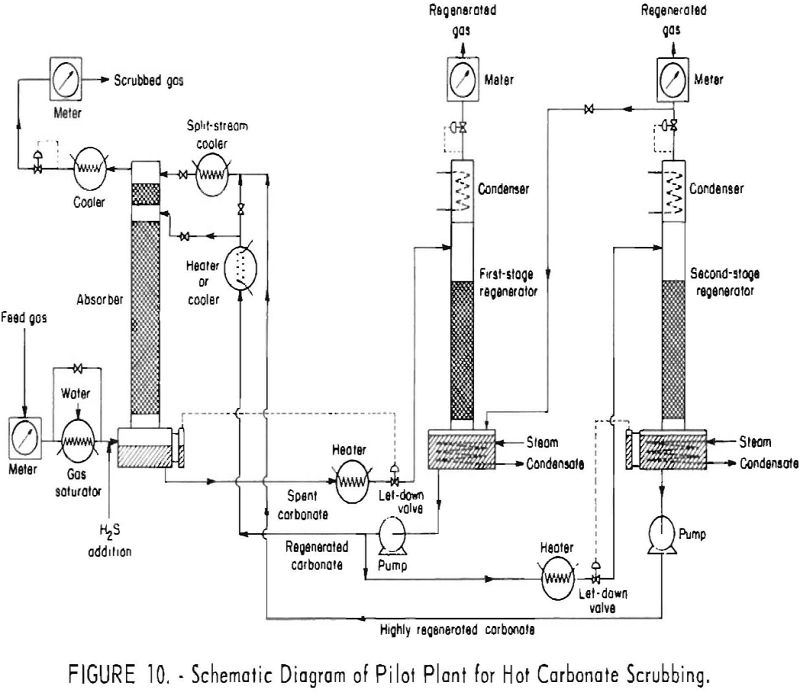
Hot carbonate purification will remove hydrogen sulfide from gas mixtures of low or high concentration of this component. The presence of some carbon dioxide in the feed gas is necessary for regeneration of the solution. Carbon dioxide aids in decomposing the bisulfide and retards formation of potassium sulfide. Carbonyl sulfide is hydrolyzed by the hot […]
Pressure Leaching of Uranium
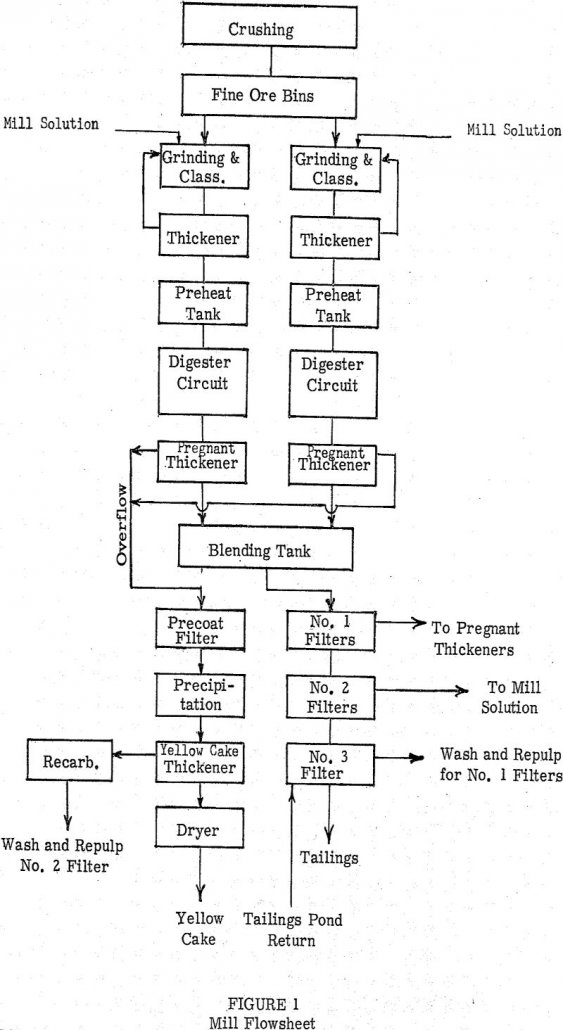
Early studies in the carbonate leaching of uranium ores led to the investigation of pressure leaching as a method to enhance the dissolution of the metal values in this system. The initial investigations conducted at the University of British Columbia supplemented by the work done by the group at the A.E.C. Grand Junction pilot plant, […]
Rare Earth Oxide Bastnasite

A bastnasite concentrate, containing about 60 percent rare earth oxides, was sulfated with concentrated sulfuric acid to eliminate the carbon dioxide, fluorine, and silica. Calcination at 1,200° F. made the gangue matter insoluble, thus permitting the separation of the soluble rare-earth sulfates. Essentially 100 percent of the rare-earth elements were recovered by this procedure. Since […]
Euxenite Metallurgy
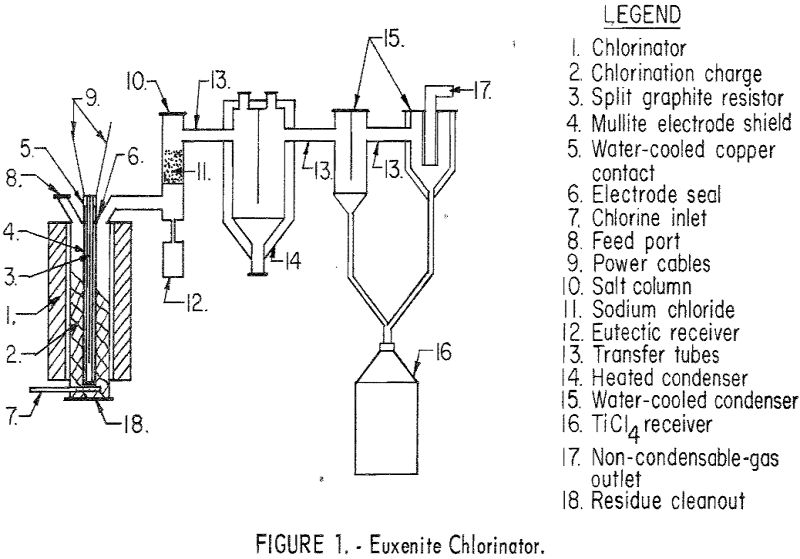
Results of this study demonstrate that multiple-oxide minerals can be processed to usable compounds by the techniques described in this paper. The chlorination procedure offers an efficient method for extracting tantalum, columbium, uranium, titanium, thorium, and the rare-earth elements from their ores. Separation of the chloride products into four groups is of additional benefit for […]
How to Make Rare Earth Chloride
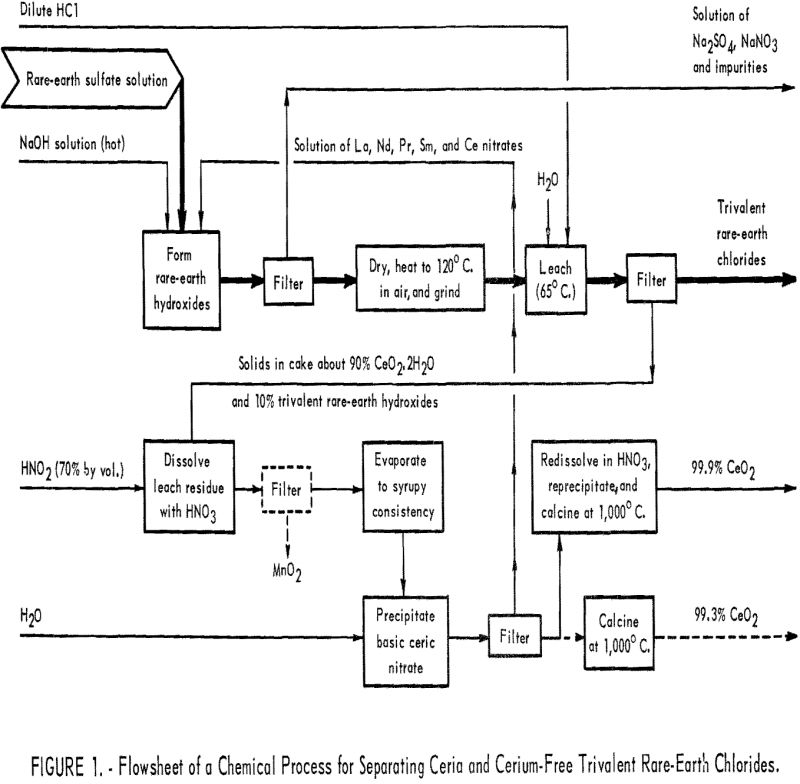
A major overall objective of the Bureau’s research program on rare-earth elements is to make ultrapure individual rare-earth metals and compounds and to determine some of their properties—properties that are not yet known or not well defined. Recognition and definition of characteristics of a rare-earth element will create new interest in it and eventually lead […]
Separating Rare Earth Elements
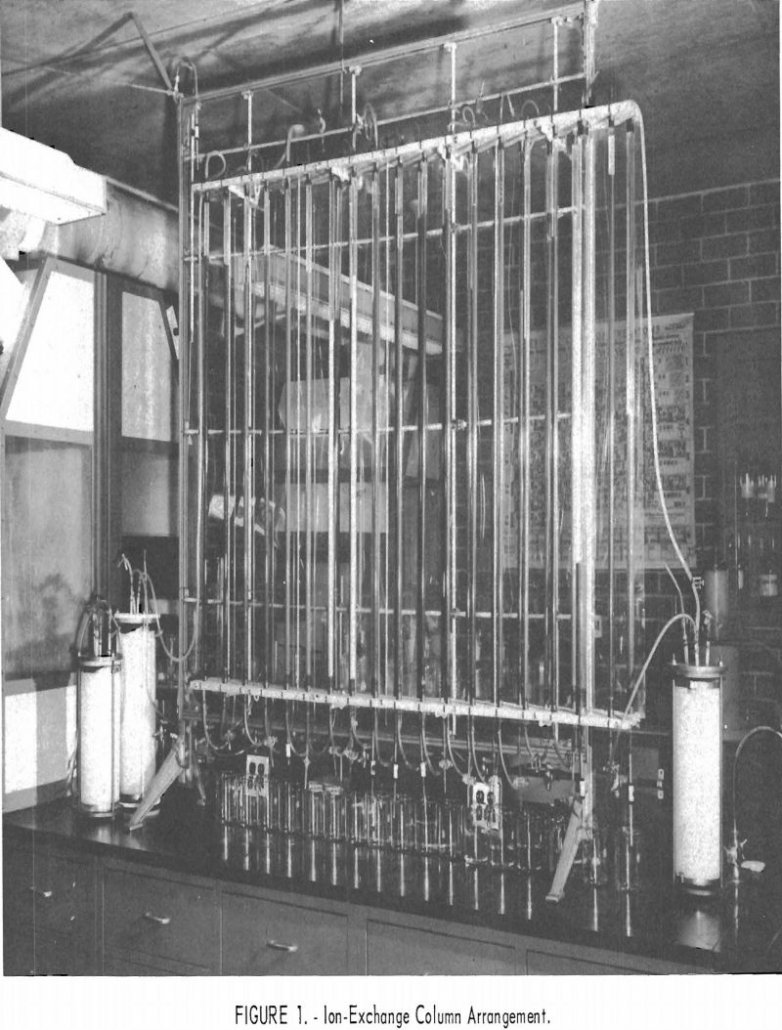
Bastnasite rare earths are now being utilized to a limited extent as a source of rare-earth materials used in the steel industry; but advances in the technology of separating the rare-earth elements from each other will make the individual elements available in adequate supply for engineering evaluation and ultimate utilization. The Federal Bureau of Mines […]
Recover Magnesium and Cadmium by Vacuum Distillation
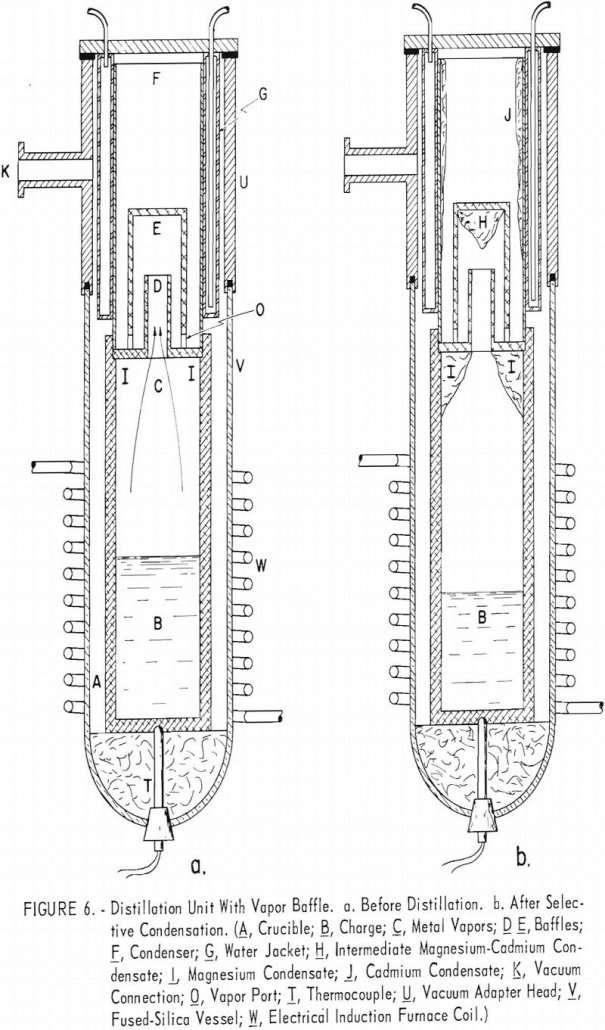
Vacuum distillation tests on a magnesium alloy containing 20 percent cadmium indicated that it would not be practicable to separate the two metals by selective vaporization of cadmium. A partial separation into products containing +90 percent cadmium and +90 percent magnesium, respectively, was effected by fractional distillation on a series of baffle plates between the […]
Recover Hydrofluoric Acid from Fluosilicic Acid Waste
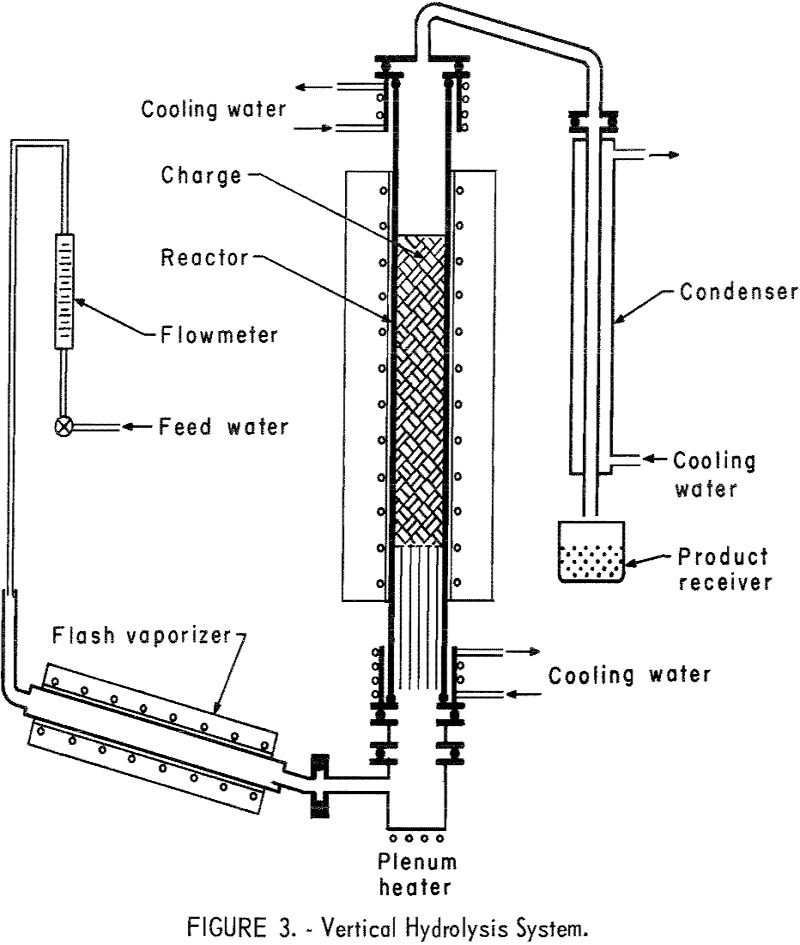
This report describes a preliminary investigation of methods for recovering hydrofluoric acid (HF) from waste byproduct fluosilicic acid (H2SiF6). Fluosilicic acid is generated by plants processing phosphate rock in the manufacture of fertilizer; most phosphate rock contains 3 to 5 percent fluorine in the form of the mineral fluorapatite (Ca3(PO4)3F). When this fluorine-bearing material is […]
Calcium Vanadate Precipitation and Processing
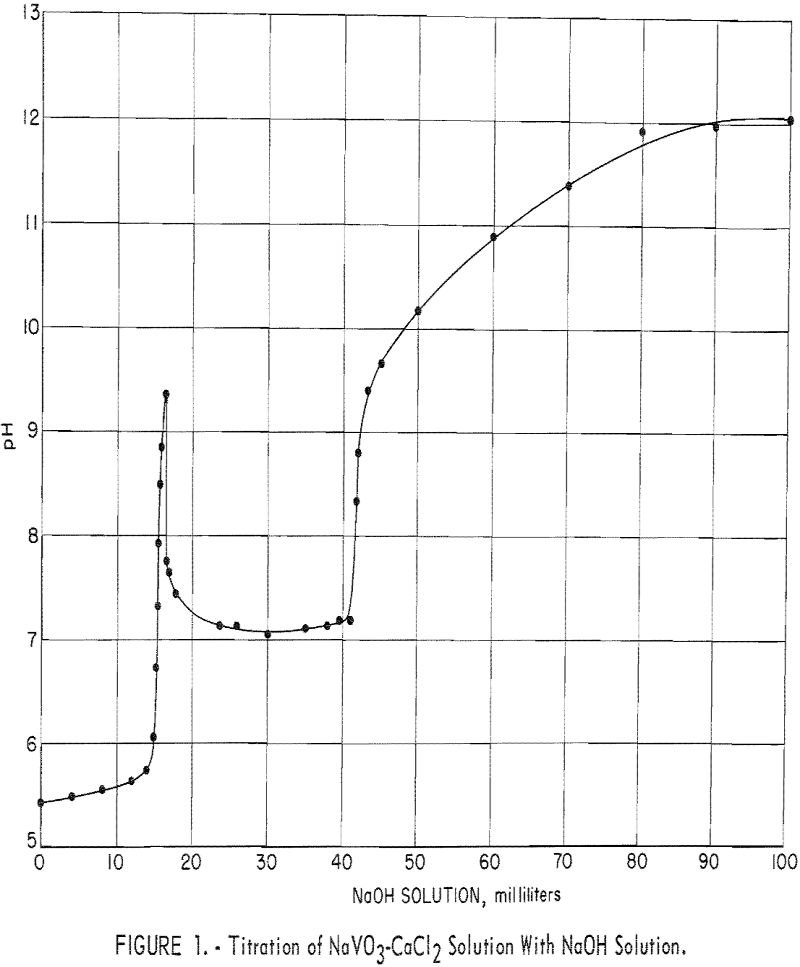
This report presents the results of a Bureau of Mines laboratory investigation to ascertain if the precipitation of calcium vanadate from low-tenor alkaline leach solutions can be practically employed for producing marketable technical-grade oxide (red cake) or ammonium metavanadate (AMV). The research was undertaken during the development of an autoclave process for extracting vanadium from […]
How to Purify & Concentrate a Manganese Leach Solution
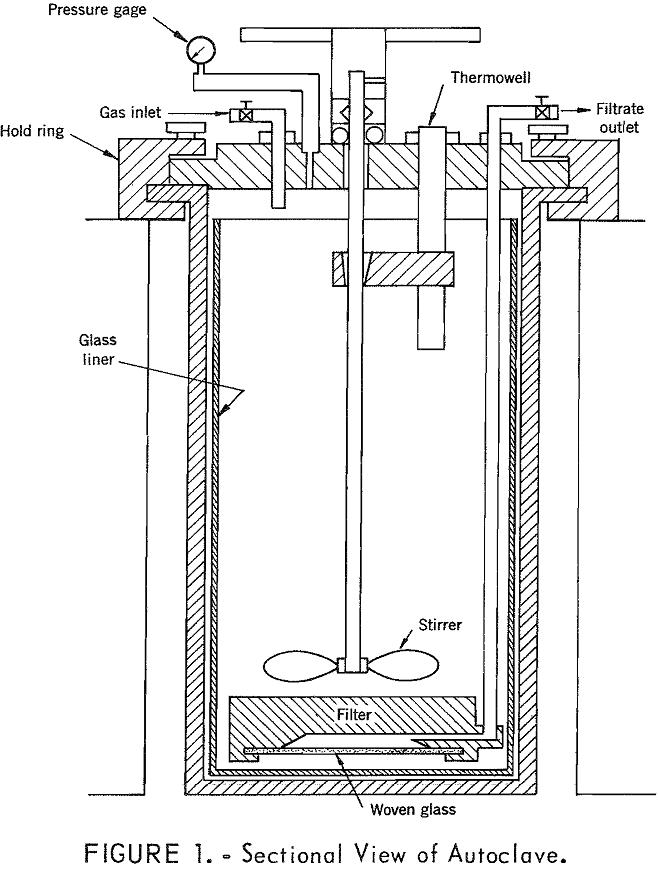
A temperature of 250° to 260° C was found to be satisfactory for simultaneous removal of iron and manganese from the pregnant leach liquor resulting from leaching of low-grade manganese ore. A holding time of about 15 minutes at this temperature was sufficient for maximum iron removal under oxidizing conditions. Using optimum conditions with oxygen […]
