Determine the Proper Grind Size for Gold Ore

Without mineralogy, estimating the optimum Grind Size for Gold Ore Sample is most conveniently made by Laboratory Testing and the agitation leaching method, and it will be necessary to make up 3 or 4 bottle charges in order to have enough ore* for the subsequent screen analysis. Take an average sample of the ore and grind […]
How to Test Ore or Rock for Gold Content

In all experiments aimed at testing the gold content of rocks or “ore”, you should ask yourself whether the conditions of his test differ in any vital particular from those that would obtain on a commercial scale; if so, there is no guarantee that his results would be borne out in a working plant. Small scale […]
Free Cyanide vs Total Cyanide Determination

It is obviously of the highest importance in controlling the action of a solution to find out what strength of cyanide and alkali it contains at any given time. Under this head are the tests for free cyanide, “total” cyanide, hydrocyanic acid, and free or “protective” alkali. Besides these cyanide constituents, which have to be […]
Method to Measure Lime Content in Cyanide Leach Solution
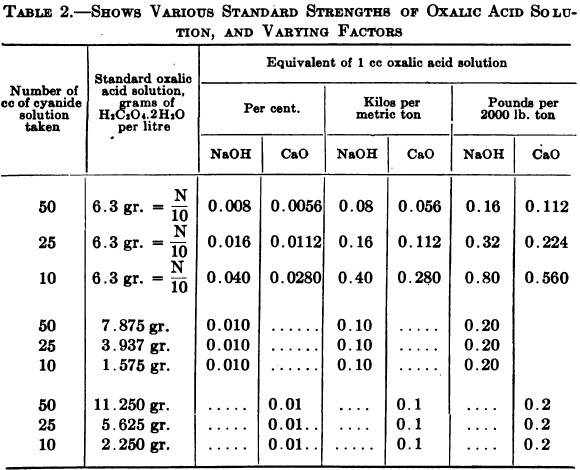
This term is usually defined as “the alkaline hydrates and half the monocarbonates” whose action is to protect the cyanide from decomposition, by acids developed in the ore and by atmospheric carbon dioxide. Either oxalic acid or a mineral acid may be used as a standard solution. Objections have been raised to the use of […]
Assay Method of Zinc in Cyanide Leach Solution

To ascertain the presence of zinc add a few drops of sodium sulphide solution to 50 cc of the solution to be tested (which should be strongly alkaline), and heat to boiling point. If zinc is present it will come down as a flocculent white precipitate. It sometimes happens that a cyanide solution will contain […]
Measure Copper Content of Cyanide Leach Solution
To assay for copper contained in a cyanide solution, the first essential step of the method is to completely decompose all the cyanogen compounds. For this purpose Clennell recommends evaporating 100 cc of the solution to be tested with 5 cc of nitric and 10 cc of sulphuric acid until dense white fumes are freely […]
Thiocyanide Thiocyanate Sulphocyanate Assay Determination
The method usually given for detection of Sulphocyanate (Thiocyanide, Thiocyanate) is to acidify and add a ferric salt, a blood-red color indicating the presence of KCNS. Silver and copper, however, interfere, and if present in sufficient amount may entirely prevent the formation of the characteristic color, so they must be removed before any determination can […]
Cyanate Determination Method
Clennell gives among other cyanate assay methods the following based on the fact that when KCNO is treated with an acid it does not decompose into HCN but reacts in the sense of the equation, KCNO + 2HCl + H2O = KCl + NH4Cl + CO2 By determining the amount of ammonium compound formed a measure […]
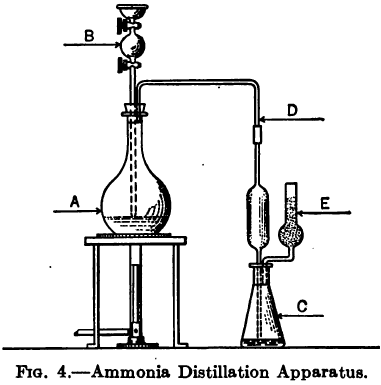
Determination of Ammonia and Ammonium Salts
The following method is given by Clennell to determine the Ammonia and Ammonium Salts content by assaying cyanide leach solutions. “Add excess of AgNO3, i.e., sufficient to precipitate all cyanogen compounds, then a little NaCl to remove excess of AgNO3, filter, wash, evaporate filtrate on a water bath to a moderate bulk, distil with caustic soda or sodium […]
Volhard’s Silver Determination & Argentometry
Volhard’s method is a type of Argentometric titration of silver content determination. The Volhard Method requires: a decinormal ammonium or potassium sulphocyanate solution, a decinormal silver nitrate solution, ferric indicator. A decinormal silver nitrate solution contains 16.988 grams of AgNO3 per litre, or 10.788 grams of Ag. It may be made up by direct weighing of the pure […]
