AMMONIA SOLUTION STRENGTH DETERMINATION
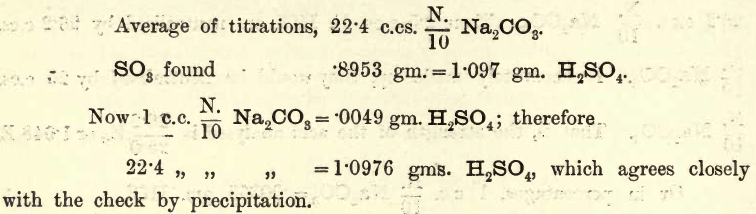
Apparatus, Reagents,—The usual volumetric and gravimetric apparatus. For analysis the student may take the bench reagent labelled 5E. NH4HO. For the standard acid pure H2SO4 of specific gravity 1.840 or thereabouts is most suitable. Pure Na2CO3 (prepared as before) is necessary for standardising the acid. Method, Reactions.—A certain volume of the ammonia solution is taken […]
METHODS OF ASSAYING

Assuming that assaying be defined as “the estimation of the commercially important elements in their ores, alloys, or products,” the following classification of methods, though not exhaustive, may be adopted for convenience. METHODS OF ASSAYING. As examples of these methods repeatedly come under the student’s notice, only a few words of explanation will be given […]
Effects of Impurities on Scorification

Method of Experiment.—A number of charges of a quartzose ore are prepared, and weighed quantities of certain substances are added to the various charges. The effects on the charge and dish are noted. Apparatus.—The muffle furnace, pulp scales, and accessories. Reagents.—Quartz, grain lead, borax glass, copper, iron, cobalt, and manganese. In place of cobalt and […]
Effect of Impurities on Cupellation

Method of Experiment.—Weighed quantities of other metals will be added to weighed quantities of silver and lead, and after cupellation the coloration of the cupel and the appearance of the bead indicate to a certain extent the impurity present. Apparatus.—The muffle furnace, balance, and accessories. Reagents, etc—Gold, silver, lead, copper, tin, antimony. Details of Experiment.—Weigh […]
Iron or Nail Method Assay Method

The Iron or Nail Method Principle: Sufficient soda is added to flux the silica, and an excess to give a fluid alkaline slag in which the FeS formed by the iron introduced and the sulphur in the pyrites may dissolve. The sulphur present acts to a certain extent as a reducer. One gram FeS2, is […]
Assay Blister Copper
This material may be taken as containing 96 to 98% copper, a few ounces of gold per ton (varies considerably with different ores), some hundreds of ounces of silver per ton, and small quantities of impurities. Though the estimation of silver has so far not been considered, the student will find it convenient to estimate […]
ESTIMATE SILVER IN GALENA

To estimate how much silver is present in galena, the student has already assayed this ore for lead, and by cupelling the buttons so obtained he will obtain some idea as to the silver contents of the ore; but to obtain accurate results the ore must be specially treated either by crucible assay or by […]
DRY ASSAY OF MERCURY ORES
The following estimation is given as an exercise in distillation. The method has been used to advantage, being convenient and requiring little apparatus. For refined methods of distillation and absorption of the mercury by gold plates consult “Beringer,” “Furman,” etc. Method. — In cinnabar the mercury is combined with sulphur. To separate these, the ore […]
Fire Clay Composition & Properties
For practice the student may take either “ Stourbridge ” or “ Dinas ” fireclay and sand. The following table gives their average composition. The Dinas sand consists almost entirely of SiO2 (free), but in the Stourbridge clay the SiO2 is mostly combined with Al2O3. The clay is preferable for practice. For reference regarding the […]
Indirect Titration

The action of permanganate of potash upon a ferrous solution is one of oxidation, hence it is evident that if any other oxidising agent is present it will count as permanganate. In such a case the titration can be used (indirectly) to estimate the quantity of such oxidising agent, by determining how much less of […]
