Table of Contents
The Bureau of Mines is developing technology which will contribute to realizing the most effective use of recycled metals. Development of improved methods for the rapid identification of aluminum alloys has been included in this program. Methods for use outside the laboratory by nontechnical personnel for the identification of wrought alloys have been of primary concern. There is no single art or method in use by scrap sorters for the identification of aluminum alloys. One of the methods which has had only limited use but which is suitable for a wide variety of applications is chemical spot tests.
There are a number of chemical spot tests for aluminum alloys reported in the literature, including combinations of tests which provide identification schemes. Many of the tests are seriously limited in their usefulness to nontechnical personnel. The tests must be simple, fast, reliable, and safe. The objective of this investigation has been to develop tests which meet these criteria. Chemical spot tests for identifying zinc, copper, manganese, and magnesium are described. Chemical tests for identifying magnesium-silicon alloys of aluminum and for identifying magnesium-base alloys are also included.
General Description
The chemical spot tests for zinc, copper, manganese, and magnesium are made on filter papers which have been pretreated with the indicators incorporated in the test solutions. Alternately, tests which do not require that the papers be pretreated can be used. If pretreated papers are used, fewer solutions are required to be carried in the field and a toxic reagent is eliminated.
A sample of material being tested is obtained by simultaneously dissolving the metal electrolytically and transferring it to the filter paper. This method, known as electrographic sampling, is illustrated in figure 1. A filter paper is wet with a suitable electrolyte and placed on a clean area of the material to be sampled. The material is connected as the anode. A cathode electrode connected to a source of voltage is contacted to the wet area of the paper, allowing a current to flow through the electrolyte in the paper. The metals are dissolved from the material and transferred to the surface of the paper in contact with the material.
The dissolved metals on the paper react with test solutions, either simultaneously with the sampling or after sampling is completed. The sample is confined to the side of the paper which was in contact with the material, and it
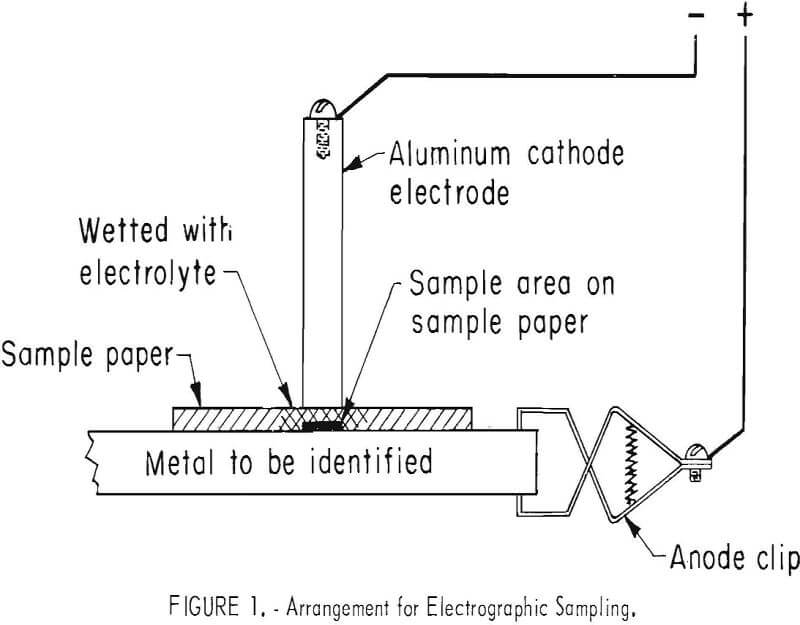
is this side to which solutions must be applied when the tests are made. The reaction products are such that colors are produced which are distinctive for specific metal ions. Two types of solutions are used electrolytes which are applied to the papers before taking the sample, and developers which are applied after the sampling is completed.
A kit containing all materials required for making the tests for zinc, copper, manganese, and magnesium, using pretreated papers, was assembled into a 4- by 6-inch plastic card file box. This kit is shown in figure 2.
The chemical tests for identifying magnesium-silicon alloys and magnesium-base alloys are not made on filter paper using electrographic sampling, but are performed by applying test solutions directly to the material.
Sampling Device
A very satisfactory electrographic sampling device is a 9-volt transistor radio battery with a current-limiting resistor and a milliammeter in series with an aluminum cathode electrode. This device is illustrated in figure 3.
A convenient cathode electrode is a piece of 3/8-inch-diameter aluminum rod about 2 inches long. Both 1100 alloy and 6061 alloy aluminum rod have
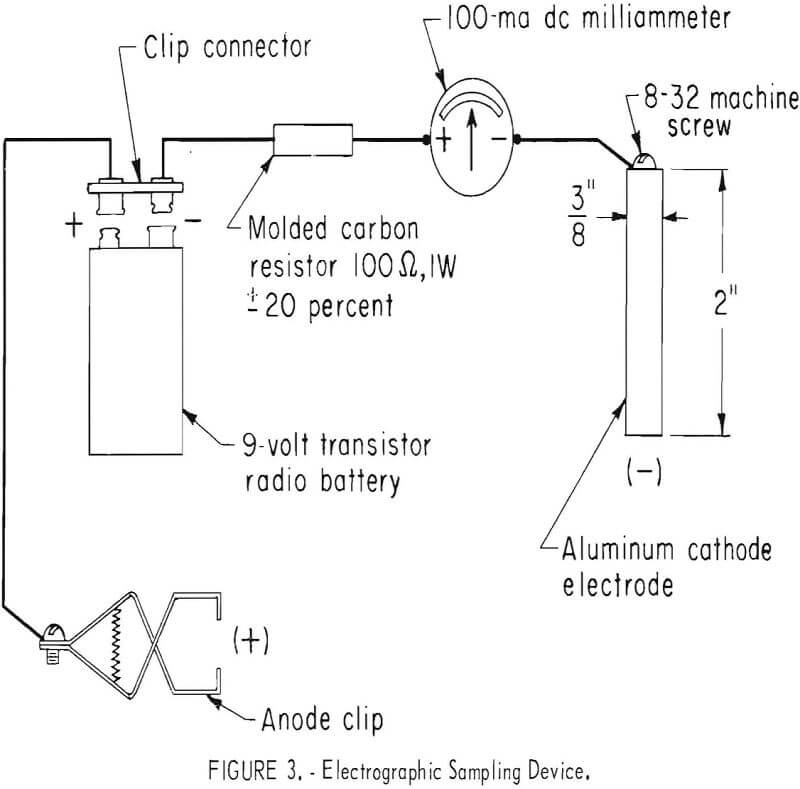
been used with no apparent difference in their performance. One end of the cathode is drilled and tapped to receive a machine screw to provide a terminus for the negative lead wire. The other end of the cathode is machined or filed to a flat, smooth surface for contacting the sample paper.
The resistance of the current-limiting resistor is such that the current density of the sample area during the sampling for most of the tests is about 400 ma per square inch. A 3/8-inch-diameter cathode has 0.1 square inch of electrode area. A 100-ohm ±20 pct, 1-watt molded carbon resistor provides a current flow of approximately 40 ma during sampling. The anode lead terminates at a battery clip which is used to maintain contact with the piece to be sampled.
It is recommended, but not essential, that a DC milliammeter be included in series with the resistor and cathode electrode. Any low-priced meter of the proper range will suffice. If a 3/8-inch-diameter electrode and a 100-ohm resistor are used, a 0- to 100-ma meter provides the proper range. This provides a means of monitoring the sampling operation and checking the condition of the battery. To check the battery, temporarily short-circuit the cathode to the anode clip. The meter will register a current flow of 80 to 90 ma if the battery is in good condition.
Sample Papers
Two kinds of filter paper are required for sample papers in the tests. Cellulose fiber filter paper is used for the tests for zinc, copper, and manganese; and borosilicate glass fiber filter paper is used for the test for magnesium. Many of the cellulose fiber filter papers commonly used in an analytical laboratory are not satisfactory for chemical spot tests, particularly when electrographic sampling is used. Only papers which are specifically recommended for use in spot tests, such as S&S No. 598, should be used. Most tests for magnesium in aluminum alloys described in the literature are performed on a spot plate using a chemical solution of scrapings of the alloy. The results are frequently indecisive. By using electrographic sampling on borosilicate glass fiber filter paper a much more decisive test for magnesium can be achieved. Reeve Angel glass fiber filter paper No. 934AH was used in all experimental work.
Procedure for Making Tests
Select or prepare a clean area on the material to be tested. If the piece has been anodized or treated on the surface in any way or if cladding is suspected, grind or otherwise abrade the surface to expose the base aluminum alloy.
Fasten the anode clip to the piece to be sampled.
Select the proper filter paper for the test.
Apply one or two drops of the required electrolyte to the center of the paper.
Lay the paper on the clean area of the piece to be sampled.
Apply the cathode electrode to the wetted area of the paper. Maintain contact for approximately 15 seconds.
Remove the electrode from the paper and remove the paper from the piece which was sampled.
Lay the sample paper, sample side up, on a piece of paper towel, facial tissue, newspaper, or other available nonmetal support.
Apply developer solutions, if required, to the sample area.
Description of Tests
Tests for Zinc
The tests for zinc using pretreated and untreated papers are completely different. The test using pretreated papers is preferred.
All organic indicators for zinc which were tried produced positive reactions with copper. It was found that when the copper was in the cuprous form and then complexed as a tartrate, murexide did not give a positive test for relatively small amounts of copper. Use of tartaric acid as an electrolyte was found to work satisfactorily. In addition, tartaric acid apparently has an anodizing action on low-zinc alloys which reduces the amount of sample obtained on all alloys except the high-zinc alloys. The murexide indicator is unstable when in solution, so pretreated papers must be used.
The mercury thiocyanate-cobalt test for zinc, which does not use an organic indicator, can be performed with untreated papers. It is specific for zinc when applied to the common aluminum alloys. However, it requires a rather strong aqueous solution of the mercury salt and involves carrying three solutions into the field. Either test will give good results.
Pretreated Papers
Indicator solution-Dissolve 0.2 g murexide in 100 ml H2O. Dip cellulose fiber filter paper into the solution. Allow to dry at room temperature. This solution must be made up within the day it is to be used to impregnate the papers. The treated papers are subject to a gradual deterioration. This deterioration is shown by fading of the purple color. The effect of this deterioration is to decrease the sensitivity of the test. The test papers have a useful life of 4 to 6 months.
Electrolyte-Dissolve 100 g d-tartaric acid in 100 ml H2O. This solution may also be made by preparing a saturated solution of tartaric acid at room temperature and then diluting 2 volumes of the solution with 1 volume of H2O. Obtain a sample as described under “Procedure for Making Tests.” Zinc is shown immediately as a yellow print of the sample area. The color clarity is improved sometimes by washing the sample area with 2 or 3 drops of the electrolyte, but this usually is not necessary. The color is permanent.
Untreated Papers
Electrolyte-Dissolve 5 g Na2CO3 (anhydrous sodium carbonate) and 4.5 g NaNO3 (sodium nitrate) in 100 ml H2O.
Zinc developer 1-Dissolve 1.6 g HgCl2 (mercuric chloride) and 1.7 g NaSCN (sodium thiocyanate) in 100 ml H2O. Allow to stand several days before using.
Zinc developer 2-Dissolve 1 g CoSO4·7H2O (cobalt sulfate) in 100 ml H2O to which 4 ml HCl has been added.
Obtain a sample as described under “Procedure for Making Tests.” Lay the sample paper, sample side up, on a piece of paper or other nonmetal material. Apply 1 drop of zinc developer 1 to the sample area, and then apply 1 or 2 drops of zinc developer 2. Development of an intense blue color in and immediately around the sample area indicates zinc. About 5 to 10 seconds may be required to develop the full color intensity. The color is permanent.
Tests for Copper and Manganese
The combined tests for copper and manganese using pretreated and untreated papers are essentially the same. With pretreated papers the indicators are impregnated into the sample papers by a prior treatment, whereas with untreated papers the indicators are included in the electrolyte solution.
Cupron is a selective reagent for copper which has been recommended and used for a number of years, including in spot tests for copper in aluminum alloys. The procedures for its use usually require three solutions-one for dissolving the sample, one for adjusting the pH, and one to provide ammonium ions for intensification of the color. The requirement for three solutions has been avoided by selecting a single electrolyte solution which gives an adequate sample and proper pH. The intensity of the color is developed by using a relatively strong concentration of the indicator rather than by adding ammonium ions, Cupron serves equally well, whether incorporated into the pretreated papers or included in the electrolyte for use with untreated papers.
Spot tests for manganese reported in the literature are generally one of three types: (1) The dissolved manganese is oxidized to permanganate, and the purple color is observed; (2) the dissolved manganese is precipitated as manganese dioxide, and the brown precipitate is observed; and (3) the dissolved manganese is reacted with oxygen and a benzidine salt or related compound to produce a reduction product of the indicator called “benzidine blue”.
The first type of test cannot be made on filter paper. The second type requires a relatively large sample to produce a decisive result. In the third type of test, the “benzidine blue” is formed by an oxidation-reduction reaction in which a benzidine-related compound, an oxidizing reagent, manganese ions, the filter paper fiber, and oxygen from the air all play a role.
Benzidine dihydrochloride and a number of compounds related to benzidine have been proposed as indicators for manganese. Most were found to be too easily reduced, thus requiring, at best, carefully controlled test conditions. The benzidine-related compound, 4,4′-methylenebis (N,N-dimethylaniline), has been selected because it is relatively unaffected by test conditions. A small amount of chemical reduction of the indicator unrelated to the presence of manganese will occur, giving a streaked light-blue tint to the surface of the paper. There is a clear contrast between this general tinting effect and the highly saturated blue produced when manganese is present.
The combining of the copper and manganese test into a single test results in some loss in the effectiveness of the manganese test due to masking of the “benzidine blue” by the copper color in some of the sample area. This masking does not reduce the brilliance and saturation of the color, but reduces the amount of area where the color can be observed. Although this effect is greater when untreated papers are used, untreated papers nevertheless provide an adequate test.
Pretreated Papers
Indicator solution—Dissolve 0.5 g cupron and 0.3 g 4,4′ -methylenebis (N, N-dimethylaniline) in 100 ml methyl alcohol or ethyl alcohol. This solution is stable when protected from long exposure to light. Dip cellulose fiber filter paper into the solution. Allow to dry at room temperature. These pretreated papers are subject to deterioration after drying if exposed to light for several hours. Deterioration is evidenced by a change of color from white to pale blue and then to a light brownish tint. No deterioration has been observed for papers stored in a box or in a black plastic envelope after more than 6 months.
Electrolyte-Dissolve 5 g Na2CO3 and 4.5 g NaNO3 in 100 ml H2O.
Manganese developer 1-Dissolve 0.8 g KIO4 (potassium metaperiodate) in 100 ml H2O. This solution may also be prepared by making a saturated solution of KIO4 and then diluting 4 volumes of the saturated solution in 1 volume H2O.
Manganese developer 2-Dilute 1 volume of glacial acetic acid with 9 volumes of H2O.
Obtain a sample as described under “Procedure for Making Tests.”
Copper is shown immediately as a yellow olive-green print of the sample area on the paper.
To test for manganese add 1 drop of manganese developer 1 to the same sample area used for the copper test, and then 1 or 2 drops of manganese developer 2.
Development of an intense blue color in and immediately around the sample area indicates manganese. It requires about 10 to 30 seconds for the full intensity to develop. The color begins to fade after a minute or so.
Untreated Papers
Equal portions of the above electrolyte and indicator solution are mixed to make the electrolyte solution for the copper and manganese tests using untreated paper. This solution should be protected from excessive exposure to light for extended periods. Any deterioration will be shown by the solution acquiring a dull blue color and by some loss in sensitivity.
Tests for Magnesium
So far as is known, no tests for magnesium in aluminum alloys have been proposed wherein the test can be performed on paper or which can utilize the advantages of electrographic sampling. The problems have been that a relatively large sample is required and cellulose fiber filter paper itself gives a positive reaction with the indicators. Glass fiber filter paper does not give a positive reaction and, because the dissolved magnesium is retained on the surface of the glass fiber paper, a small sample produces a recognizable coloration.
The tests for magnesium using pretreated and untreated papers are essentially the same. Quinalizarin is used as the indicator. Two developer solutions are required for either method of making the test.
Pretreated Papers
Indicator solution—Dissolve 0.2 g quinalizarin in 100 ml methyl alcohol or ethyl alcohol. This solution is stable, subject only to concentration due to evaporation of the alcohol.
Dip borosilicate glass fiber filter paper into the solution. Allow to dry at room temperature. No special conditions for storage are required and no deterioration of the treated paper has been observed.
Electrolyte-Dissolve 9 g NaNO3 in 100 ml H2O.
Magnesium developer 1—Dissolve 20 g NaOH (sodium hydroxide) in 100 ml H2O.
Magnesium developer 2—Dilute 1 volume of triethanolamine with 4 volumes of H2O.
Obtain a sample as described under ”Procedure for Making Tests.” Lay sample paper sample side up, on a piece of paper or other nonmetallic material. Apply 1 drop of magnesium developer 1 to the sample area. Then apply 4 to 6 drops of magnesium developer 2 to the sample area. This solution is used to wash away the film or cloud which otherwise partially obscures the characteristic color for magnesium. Development of a grayish blue print of the sample area indicates magnesium in the metal.
Untreated Papers
Equal portions of the above electrolyte and indicator solution are mixed to make the electrolyte solution for the magnesium test using untreated papers. The solution is stable, but evaporation of the alcohol may cause precipitation of the indicator. The quinalizarin is present in the solution both as a suspension and as a true solution. This can result in a deficiency of indicator in the sample area if care is not exercised when untreated papers are used. The electrolyte is applied to the sample surface of the paper, and it is important that this area, rather than areas which have been wetted by capillary action within the paper, be located directly under the cathode electrode. Use of pretreated papers eliminates this problem and makes the test results more reliable and uniform.
Other Tests
Two additional tests which may have value are a test for identifying the magnesium-silicon-base alloys and a test for differentiating between aluminum-base alloys and magnesium-base alloys. These tests are not made on filter paper. They are based on observations of the results of direct chemical dissolution of the metal.
Test for Magnesium-Silicon Alloys
A test for determining whether the material is of the 5000 series (magnesium alloy of aluminum) or of the 6000 series (magnesium-silicon alloy of aluminum) can be made by observing the action of a strong acid on the material. The test is not specific for silicon. It must be determined that the material does not contain significant amounts of zinc, copper, or manganese but does contain major amounts of magnesium, thus indicating a 5000 or 6000 series alloy.
Solution—Dissolve 25 g citric acid in a mixture of 20 ml concentrated HNO3 (nitric acid), 30 ml H2O, and 50 ml concentrated HCl (hydrochloric acid). Allow to stand 1 hour before using. The solution will remain effective for at least 6 months.
On the material to be tested, select an area which has been previously etched by electrographic sampling or, if none is available, make a test for copper or magnesium so that an etched area will be available. Mechanical abrading such as grinding is not as effective for preparing the surface as is electrolytic etching. Apply 2 drops of the acid solution to the etched area. Observe the reaction for 2 minutes or less. If the material is a magnesium-silicon alloy, the reaction will be rapid, a gray residue will cloud the solution and the surface of the material, the evolution of gas will be vigorous, and the wetted area will tend to spread. If the alloy is of the 5000 series, the reaction will be slow, the solution will remain clear, and only a relatively few large gas bubbles will form.
The copper and zinc alloys of aluminum will react similarly to the 6000 series alloys, while the unalloyed and the manganese alloys will react similarly to the 5000 series alloys. The reaction on series 6000 alloys is inhibited if they are in the solution-heat-treated or maximum-hardened condition. The reaction is also slowed if the metal is cold, as may occur in the winter. Any error in decision caused by these factors will be to classify a magnesium-silicon alloy as not containing silicon.
Test for Magnesium-Base Alloys
The manganese developer 2 solution (10 vol pct acetic acid) can be used to differentiate between magnesium-base alloys and aluminum- or zinc-base alloys. Apply 1 drop of the solution to a clean area on the material. Observe the reaction for 1 to 3 minutes. The solution will not react with aluminum- or zinc-base alloys but will react with magnesium-base alloys, producing bubbles. This reaction is not slowed seriously when performed on cold metal.
Safety Precautions
A primary consideration in evaluating the numerous tests available has been to employ procedures which present minimum hazard. However, it should be recognized that use of most chemicals involves some danger if care and cleanliness are not exercised. In addition, the indicators are organic compounds and full investigation of all potential toxic effects of many organic compounds may not have been made.
After the sample has been obtained, there is little need to physically handle the papers. They can be laid sample-side-up on some type of nonmetallic support before applying developer solutions and/or observing the results. This suggestion is particularly applicable to the tests for magnesium where a strong caustic solution is used and to the test for zinc with untreated papers where a solution of a mercury salt is used. In case some of the strong caustic solution does get onto the skin or clothing, the manganese developer 2, a 10-pct solution of acetic acid, can be used to neutralize the caustic. In the test for zinc with untreated paper, zinc developer 1 contains about 15 g/l of mercury. This is the minimum concentration for an acceptable test. Mercury is toxic and can be absorbed through the skin as well as ingested through the mouth, and particular care must be taken when handling this solution.
Although there is no known risk in using benzidine dihydrochloride and many benzidine-related compounds it is only prudent that all organic compounds be used in the safest manner possible. The solubility of 4,4′-methylenebis (N,N-dimethylaniline) in water is very low, whereas it is quite soluble in alcohol and alcohol-water mixtures. There is a greater chance of absorption of the indicator through the skin when alcohol solutions are used. Therefore; pretreated papers are recommended so that contact with the indicator in the field is limited to aqueous solutions.
Identification Scheme
The qualitative tests described can be performed in a sequence which will identify the appropriate alloy group for most of the common alloys. It is recommended that the tests be performed in the following order: (1) Zinc, (2) copper-manganese, (3) magnesium, (4) magnesium-silicon. The procedures are illustrated in figures 4 and 5.
The tests may be used in some cases to make semiquantitative estimates of the amounts of the alloying elements which are present. These estimates can in turn be used to distinguish between some of the common alloys within a group. The color for minor amounts of an element can be intensified by increasing the sampling time and/or current. Decreasing the sampling time and/or current reduces the color intensity for intermediate amounts of the element. The results of tests made on the unknown alloy are compared with those of tests made under identical conditions on alloys of known composition.
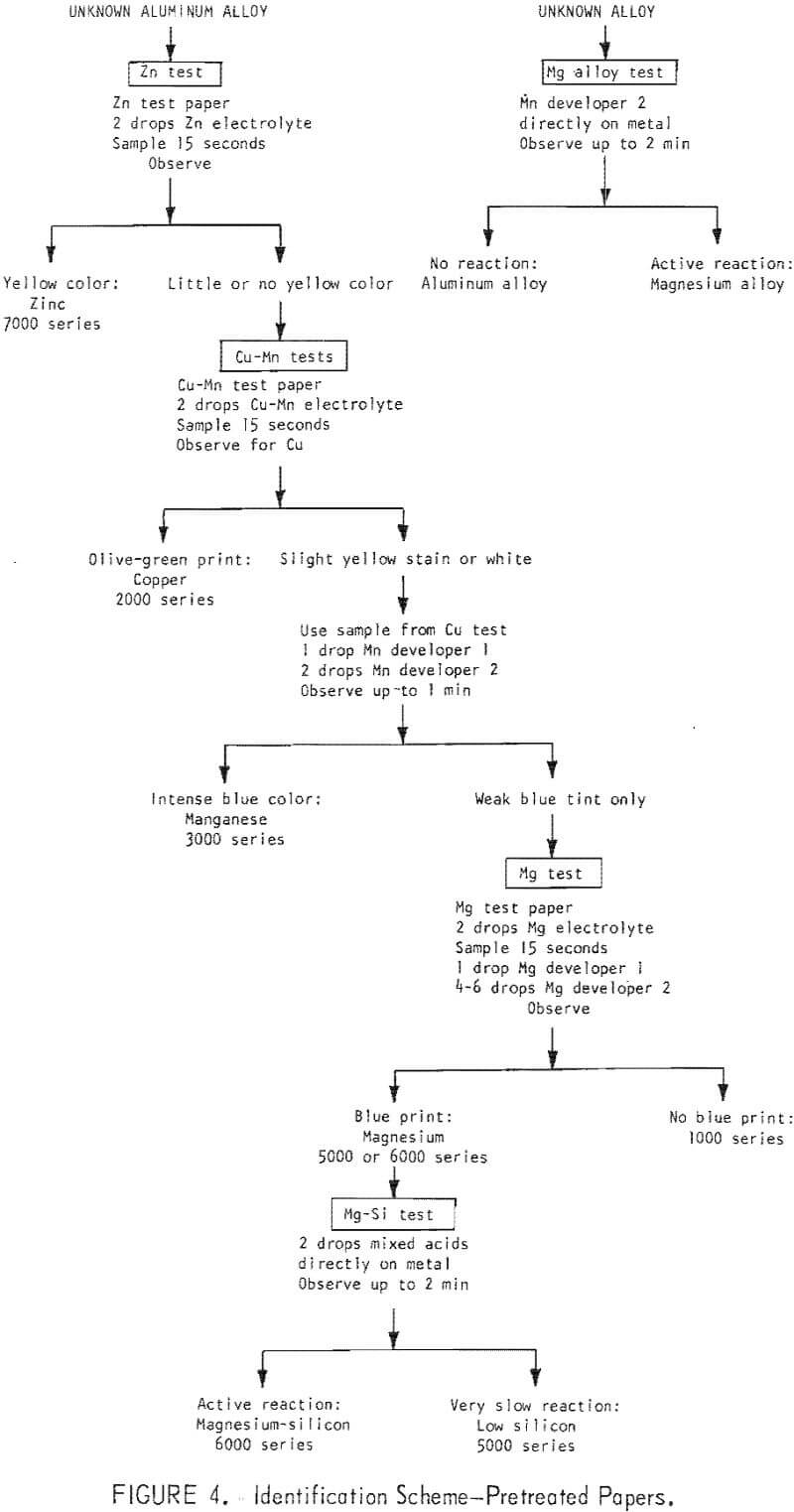
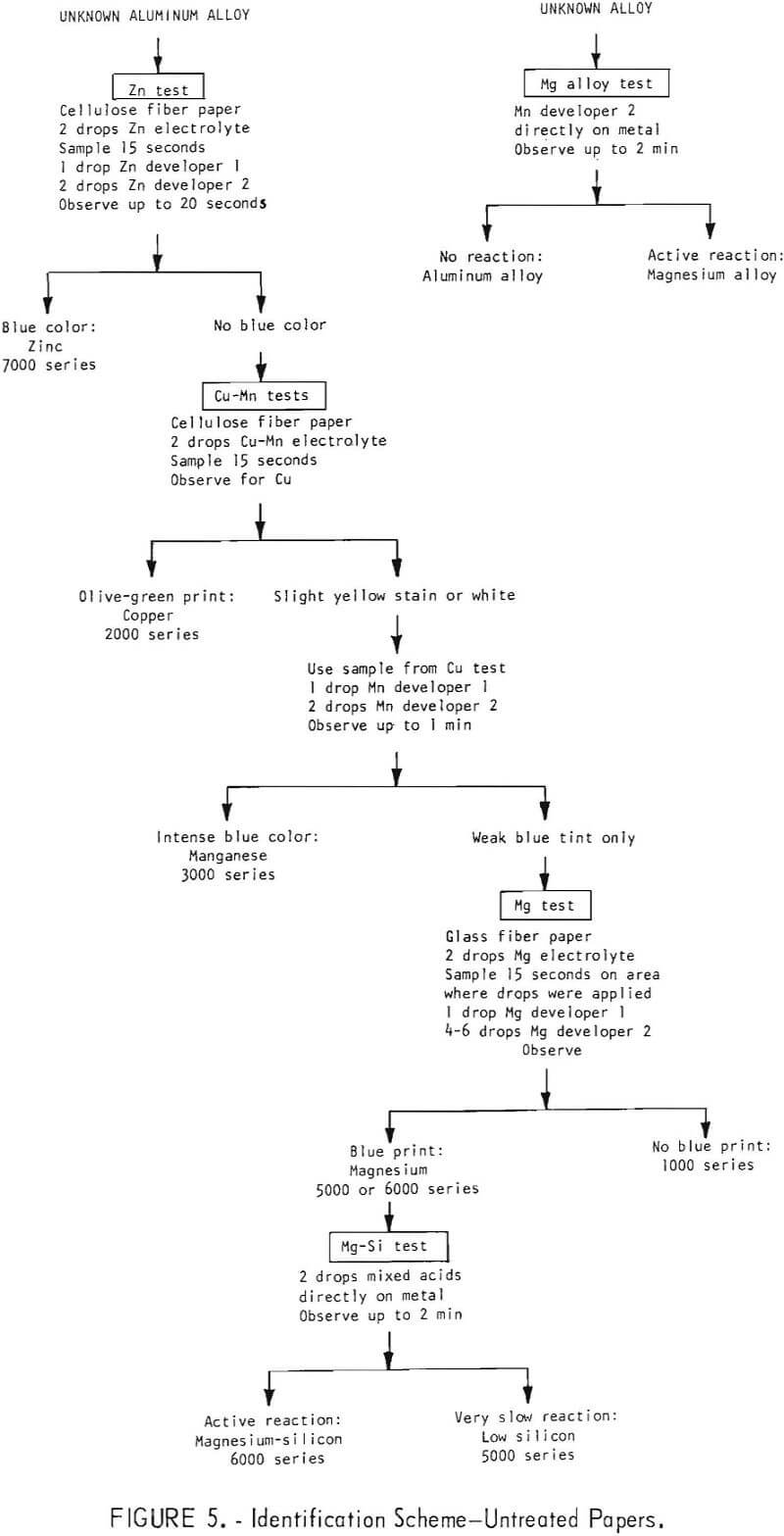
The tests for zinc, copper, and manganese give the best semiquantitative response. The test for magnesium does not provide for good semiquantitative interpretation. The results of the magnesium-silicon mixed acid test are subject to too many other -variables to be useful for semiquantitative interpretation.
Summary
Zinc, copper, manganese, and magnesium as major alloying elements in aluminum alloys can be identified using electrographic sampling and simple chemical spot test procedures. Either pretreated or untreated sample papers may be used. A test based on direct chemical solution of the metal may be used to differentiate between the magnesium alloys of aluminum and the magnesium-silicon alloys of aluminum. Aluminum-base alloys may be differentiated from magnesium-base alloys by using a test based on direct chemical solution of the metal. The tests can be performed in a given sequence to identify the alloy group for the sample piece.
