Table of Contents
Improved ceramic materials are in constant demand for processes involving severe chemical, corrosive, and thermal environments, especially at high pressures. During the past decade, demand for higher quality ceramic materials has significantly increased. This is true, for example, in the steel industry where oxygen steelmaking has increased production rates and operating temperatures, thereby compounding the demand for basic refractories that can withstand higher temperatures for use in furnace linings, ladles, stacks, checkers, etc. Since the steel industry constitutes 65 percent of the refractory consumption, efforts to meet industry’s demands for high-quality refractories have increased accordingly.
The development of chemically bonded refractories represents an important accomplishment in the advancement of the technology. Chemically bonded brick, also referred to as unfired brick, is formed with the aid of selected additives that set up at room temperature and provide structural integrity, eliminating the need for high-temperature sintering.
Chemically bonded refractories offer significant energy savings by eliminating the need for high-temperature processing. In addition, the many methods for modifying the chemical bond offer a large number of opportunities for developing new compositions to withstand a variety of severe environments encountered in many industrial processes. However, it should be recognized that chemically bonded refractories using calcium aluminate, sodium metasilicate, MgSO4 (magnesium sulfate), MgCl2 (magnesium chloride) , H2SO4 (sulfuric acid), phosphoric acid, and alkali phosphates as bonding agents have been available for many years.
This report presents a review of literature on the present state of the art of chemically bonded refractories and identifies areas requiring research and development to fulfill the need for improved ceramic materials. This work supports the Bureau of Mines’ mission to conserve the Nation’s mineral resources and reduce imports of critical materials by developing improved performance materials and using more abundant domestic mineral resources.
Chemical Bonding
Reference to a chemically bonded refractory made as early as 1905 and claimed that a valuable refractory lining could be made by “mixing such substances as magnesite, chromite, etc. with sodium silicate and calcium chloride.” Unfired refractory brick was mentioned by MacCallum and chemically bonded brick by Youngman. Progress in the chemically bonded refractories in the United States began in the 1930’s, with R. P. Heuer dominating the patent literature. Heuer received a number of patents on bonding refractory materials with sulfates, sodium silicate, sulfite lye, and small additions of clay or bentonite. In 1941, Heuer patented a chemically bonded brick that was molded in steel cases. Two U-shaped steel sheets were placed in the top and bottom of the press mold so that, after forming, the brick was encased in steel on four sides. The expansion due to oxidation of the steel casing helped to offset shrinkage at high temperatures.
Since the 1950’s, refractory research has made significant advances with the establishment of more modern laboratory facilities and the participation of scientists from other disciplines, such as physics, chemistry, and materials sciences. These scientists have brought new schools of thought to the experimentation and interpretation of research results.
Phosphate-bonded high-Al2O3 (alumina) refractories are being used in such areas .as iron-transport cars, soaking pit slag lines, and steel ladles. Various monolithic refractory linings with chemical bonding, including hydraulically cast materials, are being evaluated for use in coal conversion process vessels.
Phosphate Bond
Recognition of bonding properties of phosphoric acids and various phosphates is not new. Numerous processes for using phosphate materials as bonding agents in refractories have been known for many years. Because they possess high fusion temperatures, phosphate bonds have always been of special interest in the field of chemically bonded refractories and have been studied extensively.
Phosphate Bonding Agents
The first significant review article on phosphate-bonded refractories appeared in 1950; in it, three methods of developing chemical bonds were described: (1) reaction of siliceous compounds with phosphoric acids, (2) metal oxide-phosphoric acid reactions, and (3) reaction of acid phosphates with the refractory grains.
The reaction of siliceous compounds with phosphoric acid results in a hard white or translucent product (depending on the exact silicate composition), characterized by a lack of crystallinity. Various auxiliary materials are usually added to alter the properties of the chemical bond, but the basic setting mechanism consists of formation of a SiO2 (silica) gel. However, this low-melting frit is not a very effective bond for high-temperature applications.
A number of patents have been issued for refractories bonded with phosphoric acid. One such patent describes a ZrSiO4 (zircon) refractory with an alkaline, alkaline earth, or magnesium zirconium silicate, using HCl, H2SO4, citric, or phosphoric acid as the bonding agent. Phosphoric acid gave the best results, presumably because of its greater reactivity with the silicate components and the higher viscosity of its melts. Other silicates, such as those of Al, Cr, and Mg, react with phosphoric acid to form a chemical bond at about 200° C.
Phosphoric acid forms bonds through reactions with the cationic as well as silicate groups. For example, ZrSiO4 appears to form zirconium phosphate as well as silicon phosphates, and may form double phosphate salts of silicon and zirconium as well. Aluminum, chromium, and magnesium oxides are also known to react with phosphoric acid at 200° C to form chemically bonded materials. These metal-phosphate reaction products have been found to be refractory and stable (thermally, chemically, etc.). Instead of the oxides, the halides of Mg, Sn, Th, Ca, Ba, Al, Zr, or Ti may be used with phosphoric acid to form a chemically bonded refractory. Aluminum hydrate may be used with refractory clay, filler, and phosphoric acid to form a bond that becomes permanent when heated to 100° to 300° C.
A third method of using phosphates in refractory chemical bond formation is by the direct addition of monobasic or dibasic phosphates. Either alkaline earth acid phosphates or ammonium acid phosphates with aluminous materials may be used in place of phosphoric acids. In fact, since the reaction with phosphoric acid is very rapid, the use of phosphates of alkaline and alkaline earth metals is preferred. Even more preferable is the use of various organic derivatives such as hydrazine, hydroxylamine, aniline, methylamine, or ethylamine acid phosphates. Acid phosphates may also be formed by mixing triphosphate with an acid to form monophosphate or diphosphate. This process may be used with alkaline earth phosphates, such as calcium, which are less costly than other materials.
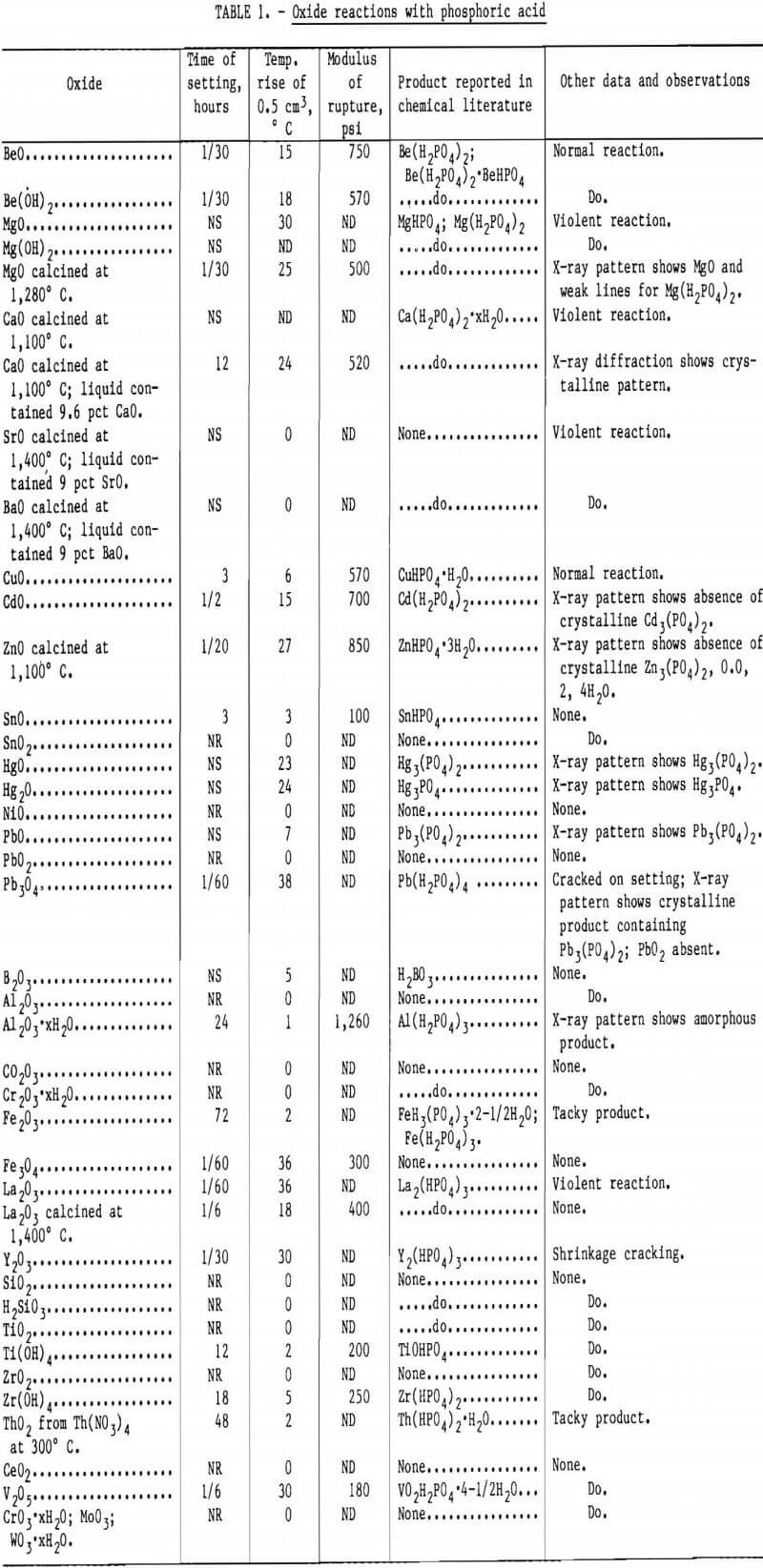
It should also be noted that phosphate bonding agents have been used for other types of applications. Sodium polyphosphate (Na4P2O7-Na6P4O13), an inorganic colloid, is used to disperse TiO2 for casting because it improves green strength. The strength of alkali silicate binders is also reported to be improved by alkali phosphate additions. A summary of oxide reactions with phosphoric acid and their reaction products is presented in table 1.
The use of alkali metaphosphates as chemical bonding agents in refractory mortars has been studied by Herold and Burst. Sodium hexametaphosphate (Na6P6O18), forms rubberlike polymers and yields high-strength mortars with fire-clay aggregates. These binders are commonly used in high-Al2O3 refractory mortars and ramming mix.
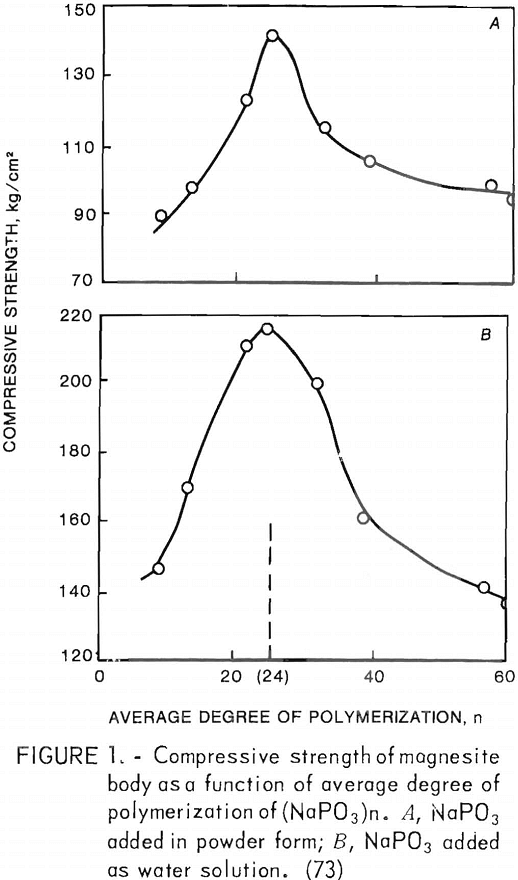
Effects of average degree of polymerization (n) of vitreous sodium polyphosphates [(NaP03)n] have also been investigated. Maximum strength was attained on samples cured at 800° C, with an average degree of polymerization of 24, as depicted in figure 1. Strength was higher when 4.3 weight-percent phosphate was added as an aqueous solution than when 5 weight-percent was added as a finely divided powder.
Fundamental Studies
Published literature describing fundamental studies of chemical bonding in refractories is almost nonexistent. However, there have been several attempts to explain the kinetic processes. Attempts to better understand bonding mechanisms, chemical kinetics of bond formation, and the conditions governing these processes have been very limited. A clear understanding of these fundamental parameters has not been achieved.
The proprietary nature of the refractories technology discourages publication, for fear of losing the competitive advantage. A large volume of patent literature exists on the subject, but emphasis is-on the-mechanics of refractory preparation rather than the science of chemical bonding or the fundamental processes.
Properties of Phosphate-Bonded Materials
With the proper selection of the bonding material and aggregates, phosphate-bonded materials do not exhibit reduced strength on heating. They remain highly refractory and possess good abrasion and slag resistance after heating. Alumina-phosphoric acid ramming compositions are particularly resistant to Fe2O3 slags at temperatures up to 1,350° C. Cement-free phosphate-bonded castables vary in their properties depending on the type and amount of bonding agent and the type and grading of the aggregate used. It is reported that tabular Al2O3-based castables show a reduction in hot strength above 800° C. This decrease becomes even more severe for castables containing MgO as a setting agent. This type of castable, however, is widely used in chemical plants because of its chemical durability. Silicon carbide (SIC) is added to phosphate-bonded high-Al2O3 products to increase their hot strength. This increase is thought to be due to formation of SiO2 phosphates in the presence of SiC.
Erosion resistance of Al2O3 castables has been improved through the use of phosphate bonding. The use of phosphoric acid is claimed to result in much higher strengths than the use of metal phosphates such as aluminum phosphate. Phosphoric acid is the preferred binder for attaining maximum bond strength and the hygroscopic tendencies of these compositions can be eliminated by curing at 650° F. High bond strength, dimensional stability, and resistance to erosion are retained to temperatures of 3,400° F in these compounds, and resistance to erosion is improved by about an order of magnitude over the existing commercial erosion-resistant castables. Stiffening and subsequent loss of workability observed in phosphate-bonded high-Al2O3 refractories is believed to be caused by the precipitation of insoluble aluminous orthophosphates forming as a result of the reaction of acid salts with Al2O3-bearing materials in the mix. The use of inhibited phosphoric acid as the bonding agent prevents this loss of workability.
The Al2O3-H3PO4 reaction is reported to have the following sequence:
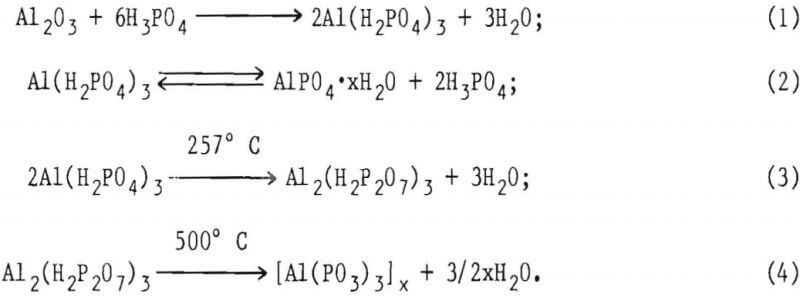
The orthophosphate Al(H2PO4)3 is water soluble and, as the bonding phase, is sticky and very viscous. It is a precursor to Al2(H2P2O7)3 and Al(PO3)3 in the cured refractory.
Prevention of softening requires stopping or slowing down the reaction described in equation 2. This is accomplished in one of two ways: The Al2O3 surfaces are coated with a nonreactive substance that prevents H3PO4 from reacting with the Al2O3, which keeps the pH low with excess H3PO4 and shifts equation 2 to the left to retain soluble acid phosphate; or a sequestering agent is used to hold the aluminum in solution to prevent AlPO4 precipitation.
The volume stability is measured either by creep under load or by reheat- change at high temperatures and is an important performance criterion in many refractory applications. The volume stability of burned and unburned phosphate-bonded high-Al2O3 brick was determined by Baab and Blackwood. The authors concluded that phosphate-bonded high-Al2O3 refractories had poor high-temperature volume stability, compared with conventionally made brick with corresponding Al2O3 contents.
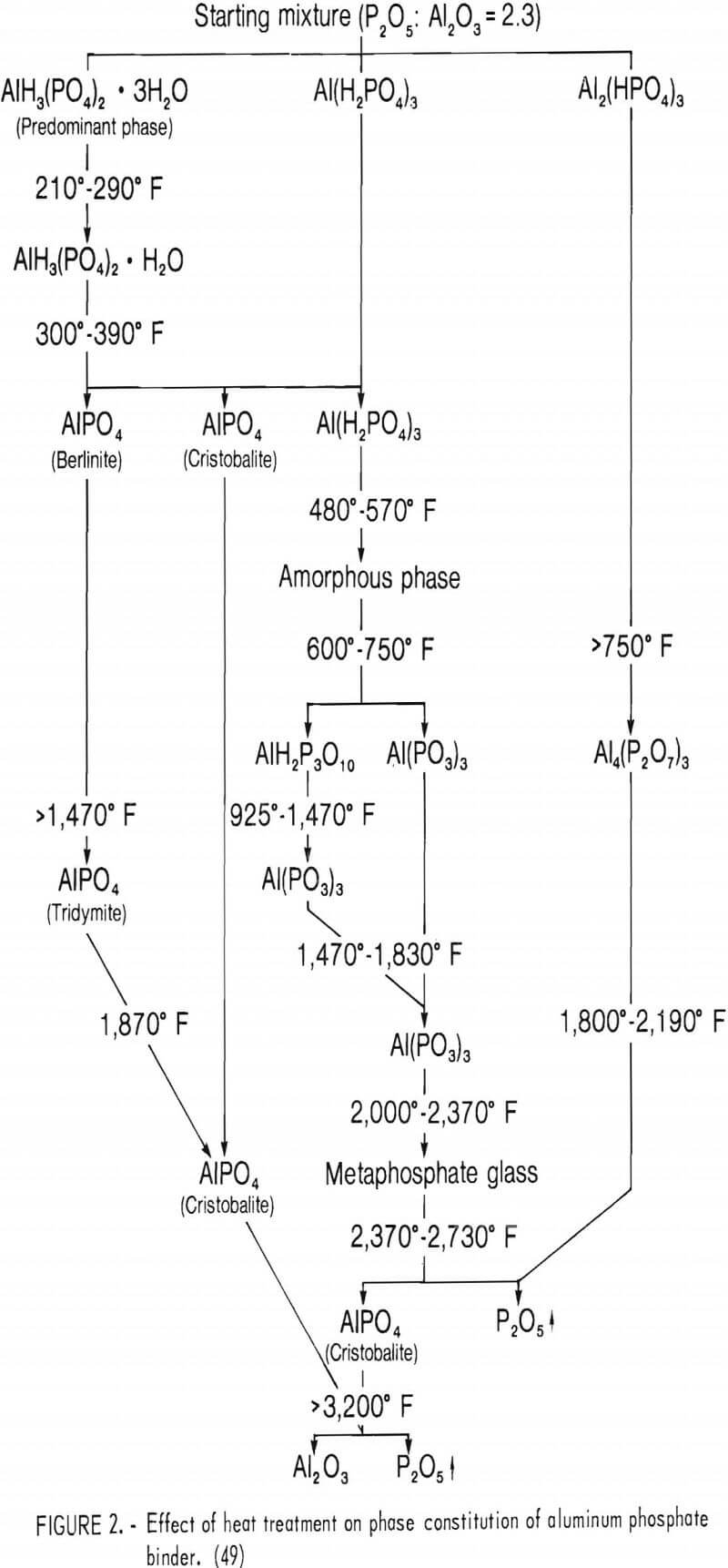
Figure 2 summarizes the phase conversions in an aluminum phosphate binder with a molar ratio of (phosphorus
pentoxide) to Al2O3 of approximately 2.3. The diagram provides a general reference for the various phases that may be produced and the approximate temperature ranges over which phase transformations or conversions take place. As shown, extensive physicochemical changes can take place upon heating the aluminum phosphate phase. It is generally agreed that the hydrated aluminum phosphate phase, AlH3(PO4)2·3H2O, is the major phase producing chemical bonding. Upon further heat treatment, this phase is eventually converted to AlPO4 (berlinite and cristobalite forms) and Al(H2PO4)3. Orthophosphate AlPO4 is isostructural with SiO2 and shows similar inversions to the alpha and beta forms of quartz, tridymite, and cristobalite. The compound Al(H2PO4)3 is a highly hygroscopic phase, which is converted to an amorphous phase above 570° F. Dehydration processes are completed between 925° and 1,470° F. A glassy metaphosphate phase appears above 2,000° F, decomposing to AlPO4, with evaporation of P2O5. The AlPO4 is reported to be stable up to at least 3,200° F before decomposing to form Al2O3 by giving off P2O5 vapors.
Hot gunning materials with phosphate binders for use in the maintenance of basic oxygen furnaces (BOF) are commercially available. Operator-controlled variables, such as moisture content and distance from the lance to the wall, contribute significantly to the performance of these phosphate-bonded gunning mixtures. Aggregates from reclaimed BOF brick containing carbon demonstrate improved adherence between the gunned material and wall, compared with conventional aggregates. Comparison of the amount of bonding agent with strength data shows that as the quantity of bonding agent increases, the cold strength increases. However, hot modulus of rupture (MOR) decreases with increasing quantity of bonding agent after an optimum 2.25 percent, for commercially available sequestered phosphate binders in basic compositions. The short-chain phosphates give the highest hot MOR. However, it should be noted that the moisture content and chain length also play very important roles in the mechanical stability of the cement. Short-chain glassy phosphates (n=7 sequestered phosphate) give optimum results at moisture levels of 3 percent, and bond levels of 2.05 percent.
Phosphate-bonded gunning mixtures (guncretes) are widely used for hot repairs of SiO2 structures in coke ovens at temperatures above 600° C, with very favorable results.
Zirconia (ZrO2) refractories with phosphate bonding agents are receiving increasing use because of their high refractoriness and low thermal conductivity. Small additions of metallic powders, such as nickel, further increase the strength and thermal shock resistance of these compositions. Rate of heating in the early stages of the curing process is a significant factor in the development of final density (porosity) and mechanical strength.
Low-shrinkage ramming compositions of high-Al2O3 bodies have been prepared from coarse-grained chamotte, clay, corundum, and phosphoric acid. Mullite (3Al2O3·2SiO2) formation by the reaction of corundum with free SiO2 is thought to account for the lack of significant shrinkage in these systems during service. Curing of these compositions at temperatures above 400° C reduces the hydration tendency of the AlPO4 aluminum phosphate bond.
Hydrated alumina (Al2O3·3H2O) reacts with H3PO4 without heat to form variscite (AlPO4·2H2O) and a mixture of amorphous products. The Al2O3 phosphate bond produced by direct incorporation of Al2O3·3H2O into the refractory body, followed by flaked lime, was found to be much stronger than those produced with Al2O3 phosphate prepared separately.
Phosphate-bonded basic refractories have been manufactured from fired MgCO3 (magnesite) with high strength and good spalling resistance. These compositions have been used as ramming mixtures for high temperature furnaces up to 1,500° C.
MgO + 2H3PO4 → Mg(H2PO4)2 + H2O…………………………………………………….(5)
Forsterite refractories with magnesium phosphate bonds have shown increased strength at temperatures between 500° and 700° C, and no signs of diminishing strength to 900° C. Refractories made with about 5 percent bonding agent exhibited the highest compressive strengths. Increasing the chemical bonding agent beyond 5 percent decreased the strength because of a “washing out” of the excess bonding agent, which did not react with the refractory matrix.
Refractory brick produced from dense briquettes without chemical bonding agents have lower strengths than do porous briquettes containing 5 percent bonding phase. This phenomenon is explained by potential displacement of the bonding phase to grain boundaries without penetration through the grains to form an effective chemical bond.
Silicate Bond
Sodium silicate and ethyl silicate [(C2H5)4 SiO4] are the most common silicate binders used in refractory applications. Sodium silicate binders have been studied and used most extensively in refractories and foundry applications.
Alkali Silicates
Alkali silicate binders, especially sodium silicates, have been used in the formulation of protective coatings for refractory linings, refractory ceramic foams, waterproof cement, metal casting molds, refractory castables, and ramming mixtures.
Refractory compositions in which alkali silicates have been used as chemical binders include high Al2O3, Al2O3 silicates, mullite, magnesium, and several nonoxide refractory materials. Water glass (sodium metasilicate) has been used as a refractory binder for blast furnace slags , sand-clay mixtures, and other metallurgical slags.
Patent literature indicates that alkali metal silicates have been employed as refractory binders, usually with several other additives such as strengthening agents, components to provide hydration resistance, and plasticizers. Boric oxide (B2O3) or B2O3-producing compounds such as Na2B4O7 (sodium borate) or similar inorganic salts are commonly used with alkali silicate bonding agents. The main function of B2O3 is to prevent hydration and extend the shelf life of the binder. Alkali silicates have been used in refractory mixtures containing mullite whiskers and powder, Al2O3 whiskers and powder, magnesium grains, Al2O3 cements, clay concrete, and various other Al2O3 silicates. It is also reported that Na2SiF6 (sodium fluosilicate) is used frequently with water glass in cast- able refractory compositions. The addition of metal powders such as Fe, Cr, and Ni increases strength at high temperatures.
Silicate bonding agents have also been employed with phosphate bonding agents in castable formulations. Refractory castable compositions, for example, have been formulated containing sodium silicate, sodium carbonate, and Al2O3 phosphates. The use of silicate and phosphate bonding agents together has been the exception rather than the rule.
The use of gypsum (CaSO4·2H2O) in refractory compositions containing lime, calcium silicates, and dolomitic lime with water glass greatly retards the hydration of CaO and MgO in the calcium silicate solutions. The addition of 3 to 5 percent gypsum in such compositions increased the strength by 33 percent. However, gypsum contents above 7 percent reduced the strength of the calcium silicate refractories sharply.
Water glass has been most successfully used as a bonding agent in foundry applications. The chemical bonding agents used in steel foundry molds include furane binders (such as urea formaldehyde or phenol formaldehyde solutions to which furfuryl alcohol has been added) with 5 to 20 percent P2O5 by weight of the furane binder.
Ethyl Silicate
Ethyl silicate-bonded refractories are prepared from a slurry of refractory grains with ethyl silicate, containing amine additives. The slurry is made as dry as possible and poured, tamped, or pressed into a vibrated mold. When the slurry has gelled, the article is stripped from the mold and the volatiles are removed by air drying and baking the pressed block to 200° C.
The use of ethyl silicate as a binder in refractory components is also discussed in the refractories literature. The relatively good performance of nozzles of mullite and ZrO2 with calcined Al2O3 compositions in sliding gate systems has been attributed to the use of ethyl silicate bonding agents. The ability of ethyl silicate-bonded refractories to withstand the combined effects of severe thermal shock and chemical corrosion is closely related to the fine texture of the Al2O3, matrix in the refractory.
Ethyl silicate binders are especially appropriate for the formation of multilayered refractory molds in the lost-wax process. Multi-layered molds have been prepared using refractory grog or powdered fused quartz fillers and ethyl silicate binders. However, the hydrolysis and condensation of ethyl silicate can affect the quality of the refractory products fabricated.
Other organic silicate binders have also been prepared by reacting sodium silicate with ethyl silicate. The time of setting for the organic silicate formed by this reaction at room temperature is about 90 to 100 minutes, enabling the product to be formed before setting occurs. Compressive strengths as high as 400 kg/cm² have been obtained using these organic silicate binders.
When ethyl silicate is used as a refractory binder, it is usually prepared by the direct reaction of silicon tetrachloride and ethyl alcohol. If the alcohol is anhydrous, the product is an orthosilicate (tetraethoxysi lane), with HCl gas being produced as a byproduct:
SiCl4 + 4Et·OH → Si(OEt)4 + 4HCl……………………………………………………..(6)
However, if industrial ethyl alcohol, which almost always contains some water, is used, the product obtained, called technical ethyl silicate, is a mixture of the orthosilicate (tetraethoxysilane) and polysilicates (ethoxypolysiloxanes), because the water present in the alcohol causes some hydrolysis and polymerization. When used by itself, ethyl silicate has no bonding ability and, therefore, it is necessary to treat ethyl silicate with water to form a gel from the resulting ethyl silicate hydrolysate, which is the actual bonding agent. Alkaline hydrolysis procedures are in general preferred when ethyl silicate is used in the manufacture of refractories. However, acid hydrolysis procedures are usually preferred in foundry processing. The water for the hydrolysis of ethyl silicate can be provided by a SiO2 aqua- sol, and in this way a hydrolysate with a high SiO2 content can be prepared.
By using strongly basic amines with the ethyl silicate, intricate refractory shapes can be cast to close tolerances. A few examples are electric furnace element carriers, crucibles, and glass feeder ware, such as plungers and orifice rings. Most refractory materials are suitable for use with mixtures of ethyl silicate and highly basic amines (amine-modified ethyl silicate). Included among the frequently used cast- able refractory materials are Al2O3 and Al2O3 silicates such as sillimanite and mullite, ZrO2, ZrSiO4, and SiC. Finished products with these compositions have high dimensional accuracy and excellent surface finish, as well as good resistance to thermal shock.
Oxychloride, Oxysulfate, and Oxynitrate Bonds
Magnesium oxychloride cement is the product obtained when MgO and solution of MgCl2 react together. Magnesite is calcined so as to give a lightly burned reactive product which is ground and mixed as required with a strong solution (about 20 percent anhydrous salt) of MgCl2. Combination of MgO and MgCl2 takes place with the evolution of heat resulting in the formation of magnesium oxychloride (3Mg0·MgCl2·nH2O). The aged oxychloride cement appears to be composed of varying-sized particles of Mg(OH)2 (magnesium hydroxide) from which radiate a large number of fine needle like crystals of oxychloride, which bond the material together.
Addition of MgCl2 solution to MgO powders provides appreciable strength through the formation of cementitious phases at the grain boundaries. The dissociation of the bond phase occurs over a wide range of elevated temperatures, with loss of water at lower temperatures and loss of HCl at higher temperatures, leaving only MgO as the residual phase. The system MgO-MgCl2-H2O has been the subject of numerous investigations since the discovery of the hydraulic properties of MgO and MgCl2 mixtures in water during the 1800’s. The compounds 5Mg(OH)2·MgCl2·nH2O and 3Mg(OH)2·MgCl2·nH2O have been identified as the cement-forming compounds.
A similar magnesium oxysulfate cement is used as a binder in many structural materials and for refractory applications. Solutions of MgSO4 react with active MgO to form the cementitious phases, 3Mg(OH)2·MgSO4·nH2O and 5Mg(OH)2·MgSO4·nH2O, identified as the stable phases at 25° C, with other phases formed at higher temperatures. Other analogous mixtures, such as zinc and aluminum oxy-chlorides, have also been studied and are used in a limited number of applications. Aluminum oxychlorides are excellent binders for refractory aggregates at temperatures to 1,500° C. Oxybromide analogs of magnesium and aluminum oxychlorides have been prepared, but little information is available regarding their properties.
Magnesium oxychloride and magnesium oxysulfate cement compositions have been the subject of numerous patents. In most of the compositions suggested for refractory lining repairs, large quantities of hydrophillic colloids are used to increase the consistency and allow additions of sufficiently concentrated MgCl2 or MgSO4 solutions to the dry mix, in order to exceed the critical MgCl2- or MgSO4-MgO ratio necessary for the development of a wet mix that can be applied by brushing or troweling.
Good chemical bonds have also been obtained using nitrates [NaNO3 or Ca(NO3)2] in quantities of 8 to 20 percent by weight of solids, with a variety of constituent combinations of MgO, ilmenite, chromite ore, and Fe, Si, and Al in lesser amounts. In these compositions, nitrates react quickly with Fe-Si, forming a silicate bond. Calcium nitrate Ca(NO3)2 is preferred to NaNO3 since a more refractory silicate is formed.
Increasing the high-temperature mechanical strength of cast Al2O3 refractories by introducing organic additives such as polyvinyl alcohol, sucrose, and flour has not been very successful. The development of an organic film on the Al2O3 particles is thought to mask the intermolecular attraction forces and lower the strength of the cast refractory. Additions of up to 10 percent Al2O3 treated with HCl solutions have significantly improved the strength of Al2O3 castings at temperatures above 1,000° C. It is reported that the formation of aluminum oxychloride bond on the surfaces of γ-Al2O3 particles treated with HCl solution produces higher strength.
The dissolution of MgO from the complex is essential in the hardening of both chloride and sulfate cements of magnesia. Setting processes involve formation of Mg(OH)2 for sulfate cement and formation of basic MgCl2 for chloride cement. The agglutination of the fine particles in the cement mixture is explained by hydrogen bonds acting directly between the OH groups of the Mg(OH)2 in one case and of the basic MgCl2 in the other.
The setting of chloride cements can best be illustrated by the following chemical reaction where 3Mg(OH)2·MgCl2·nH2O forms as the bonding agent:
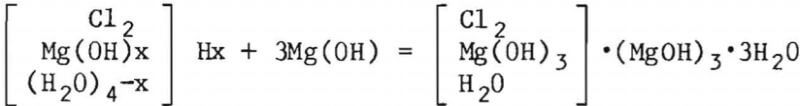
The use of the so-called “salt phase” as an inherent body component is a new element in the development of manufacturing procedures for lime-base refractories. The salt phase is mainly CaCl2 (calcium chloride), which melts at 772° C but can be lowered by as much as 400° C in the presence of other salts. The salt phase melts at low temperature, yielding a reactive liquid of low viscosity, and leaves the system gradually as a result of high-temperature hydrolysis. The formation of 4CaCl2·Ca0 upon heating and its effects on the subsequent ceramic processes is thought to be responsible for the development of a unique micro-structure and the high-temperature volume stability. The volume stabilization is believed to be helped by the progressive evolution of the HCl resulting from the high-temperature hydrolysis of chloride salts. An even more pronounced effect on volume stabilization has been observed in bodies with CaCO3 additions (along with CaCl2), the so-called calcite brick. As more gas phase (CO2) is created by the decomposition of the carbonates, and if the viscosity of the melt is increased (by addition of silicates), a marked expansion of the products may occur.
The strength of unfired refractories containing magnesium oxysulfate, magnesium oxysulfate-H3BO3 (boric acid), and sodium polyphosphate bonds has been determined as a function of temperature. All the bonding agents develop higher strength in the presence of chromite, and the addition of H3BO3 with MgSO4·7H2O increased the strength of the refractory in the 400 to 900° C range. Above 1,000° C, the strength of these same refractories was significantly decreased due to incongruent melting of magnesium metaborate.
One of the problems encountered in the use of MgCO3 refractories is the partial hydration of MgO in the presence of water. The thermal decomposition of Mg(OH)2 upon heating to 400 to 500° C and the consequent evolution of water vapor cause severe thermal spalling. Additions of approximately 1 percent B2O3, yielding material such as H3BO3, reduce the hydration tendencies of the MgO refractories. In the presence of MgSO4 or MgSO4- yielding material, the addition of H3BO3 is not only ineffective in preventing MgO hydration but actually increases the degree of hydration significantly under certain conditions. An improved chemical bond that at the same time prevents MgO hydration has been described by Martinent. The bonding agent consists of 35 mesh dead-burned MgO, from 0.5 to 5.0 percent magnesium sulfate heptahydrate by weight of MgO, and a boron compound yielding B2O3 upon firing, to provide a weight ratio of MgSO4:B2O3 of 2:1 or less. This bonding composition is used in amounts of from 10 to 60 weight- percent of the total refractory composition.
A patent by Montague describes a method for obtaining superior chemical bonding in refractory compositions containing olivine [(Mg, Fe)2 SiO4]. The olivine fines are slurried with water, and then H2SO4 is added and mixing is continued. The reaction generated produces large quantities of steam rapidly, and the mixture becomes very viscous and hardens into a solid cake. Ordinarily, the cake is crushed and screened, for convenience. Refractory linings of olivine, MgO, and chrome with the described bonding agent were found to be superior to similar compositions using sodium silicate bonding.
Conclusions and Recommendations
Chemically bonded brick offers promise in a number of refractory applications for iron and steelmaking, glass manufacturing, high-temperature chemical processes, and energy conversion processes, as well as in nonrefractory applications. Unfortunately, the efforts to explain chemical kinetics and mechanism of bond formations have been limited. With the exception of information on dental cements, few data regarding the bonding reactions and bond mechanisms are available; in addition, the identified references about bonding mechanisms are very limited. Chemical kinetics and important reaction parameters have not been systematically studied.
The possibility of forming a large variety of chemical bonds is great, thereby extending the potential applications for chemically bonded brick in severe environments at moderately high temperatures. Coal gasification and liquifaction present one area of potential applications where the thermal conditions are moderately severe (1,100° C), and high chemical durability is required for refractory liners in reducing or oxidizing atmospheres with corrosive gases and liquids.
The feasibility of using raw materials of marginal purity, such as spent refractory linings and byproduct slags, could be enhanced through the development of chemical bonding agents with various compositions for use in high-temperature environments.
Based on the conclusions outlined above, a number of research development projects are recommended:
- Fundamental research efforts should be devoted to better understanding chemical bond development for various refractory systems. Kinetics and the mechanism of chemical bond formation should be examined. A fundamental understanding of the processes leading to chemical bond formation will identify opportunities for development of materials with new and improved performance, which in turn would help conserve the Nation’s mineral resources.
- Research efforts should be devoted to developing more versatile and inert chemical bonds in chemical binder systems and combinations of binders. Attempts should also be made to determine mechanistically the role of each component in a binder system. In addition, the effects of important manufacturing parameters, such as curing rates, moisture content, and mixing methods for different binder compositions, should be determined. The role of metal powder additions should also be investigated, and the use of chemical bonding agents in combination should be explored.
- Research activities for the development of monolithic refractories should continue and be expanded to include chemically bonded compositions in addition to hydraulic bonds.
- Research should be conducted on the development of chemically bonded refractories from spent refractories, waste linings, and raw materials of marginal purity.
- Research efforts should be directed at improving the short and unpredictable shelf life of many chemical binders, which would prove very helpful in the development of next-generation chemically bonded refractory products.
- The opportunities for application of chemically bonded refractories can be greatly extended by solving certain pressing problems, such as bond migration, bloating, and low hot strengths, which have greatly limited their use.
- Pitch and tar bonding agents present some health and environmental problems; chemical bonding agents should be developed to substitute for these organic bonding agents.
- In many cases, the literature evaluation of refractory compositions has not included the service conditions to which the refractory would be subjected. It is recommended that any evaluation program following a development effort should consider the service conditions, and appropriate evaluation procedures should be instituted as part of all refractory development studies.
Related: 12 Best Glues for Metal
