Table of Contents
Low-temperature chlorination of three types of ferrochromium at 340° to 525° C chlorinated an average of 89 percent of the chromium and 95 percent of the iron contents of the feed materials. From 81.9 to 91.6 percent of the iron was removed by sublimation as FeCl3. The reactor product was a free flowing material which could be poured from the reactor. The soluble chromium chloride was recovered by leaching and filtering the reactor product to remove free carbon and unattacked ferrochromium. From 82.4 to 93.1 percent of the chromium content of the ferrochromium was recovered in the leach solution. The technical feasibility of upgrading or recovering chromium from low-grade ferrochromium by a low-temperature chlorination process has been demonstrated. Further investigation would be necessary to determine the economic feasibility and optimum operating conditions for this process.
The Bureau of Mines has engaged in a research program on the development of methods to utilize the chromium resources of the United States. The Stillwater Complex in Montana comprises the largest domestic source of chromite. Previous work by Rosenbaum, Lloyd, and Merrill has shown that chromite concentrates from the Mouat deposit in the Stillwater Complex can be smelted into a high-carbon ferrochromium containing approximately 55 percent chromium, 32 percent iron, and 7 percent carbon. The product was similar in composition to some of the high-carbon ferrochromium prepared by Hunter and Banning from Mouat concentrates.
The steel industry uses ferrochromium containing 65 to 70 percent chromium for chromium additions to molten steel. To prepare ferrochromium with this chromium content, chromite having a chromium-to-iron ratio of 2.8-3.0 to 1 must be used. The average chromium-to-iron ratio of Mouat chromite concentrates is 1.7 to 1. Ferrochromium made from Mouat chromite is considered substandard because it only contains approximately 55 percent chromium.
The object of this investigation was to make a preliminary examination of the feasibility of a low-temperature chlorination process to effectively remove the bulk of the iron content of the ferrochromium produced from Mouat chromite concentrates. By removing the bulk of the iron, the chromium con¬tent of the ferrochromium would be upgraded and could be utilized.
Data on the chlorination of ferrochromium are scarce. Devyatovskaya and Vilnyanskii treated ferrochromium with chlorine at 950° to 1,000° C and sublimed a mixture of chromic chloride (CrCl3), chromous chloride (CrCl2), and ferric chloride (FeCl3). Teterevkov and Vilnyanskii treated ferrochromium with hydrogen chloride at 600° to 1,000° C and obtained a molten chromium chloride product. The chlorination of chromite ores has been reported by Maier, and Doerner has thoroughly covered the thermodynamics of the chromium chlorides.
A 340° to 525° C temperature range was selected for the tests reported here to prevent formation of molten chromous chloride (melting point 815° C), to prevent sublimation of chromic chloride (sublimation point 947° C), to enhance sublimation of ferric chloride, and to minimize corrosion of the test equipment. Chlorination at temperatures above 525° C would result in excessive corrosion of the nickel reactor. Since FeCl3 starts to melt and sublime at 300° C, chlorination at the temperature range selected should volatilize a large proportion of the iron present in the ferrochromium, leaving most of the chromium chlorides in the chlorinator. One of the major difficulties encountered by other investigators of the chlorination of both ferrochromium and chromite was the formation of molten chromous chloride which caused plugging of the chlorinator and low recoveries, because the unreacted material was coated with the molten chromous chloride. Low-temperature chlorination would prevent the formation of molten chromous chloride and eliminate this difficulty.
Experimental Procedures
Apparatus
Preliminary tests made with a static-bed chlorinator showed that the bed became progressively less pervious to gas passage as the chlorination proceeded. The ferrochromium particles swelled as the chromium changed to chromium chloride. This swelling of the bed caused frequent rupture of the reaction tube. A rotary chlorinator was designed to overcome these problems and to permit better gas-solids contact than the static-bed chlorinator allowed.
The final apparatus (fig. 1) consisted of a 4-inch-diameter by 5-inch- long nickel reactor tapered on each end, which was rotated within an
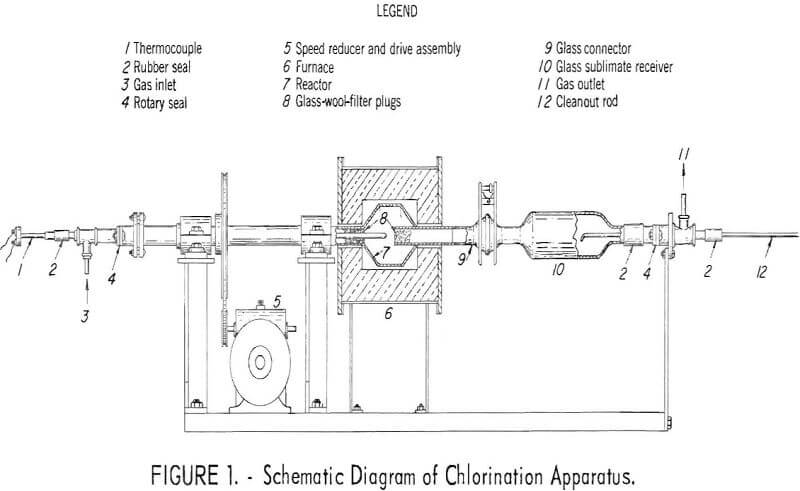
electrical resistance furnace by means of a tubular trunnion supported on bearings and turned by a pulley and belt attached to a motor-driven speed reducer. The chlorine gas entered through the trunnion. The ferric chloride produced in the reactor vaporized and was condensed in a Pyrex glass sublimate receiver attached to the reactor outlet by means of a tapered Pyrex glass connector. The glass connector taper joint was wrapped with Teflon tape where it fitted into the corresponding metal joint of the reactor. This formed a gas tight seal and prevented the expansion and contraction of the metal from breaking the glass. Rotary Teflon-packed seals, operated at room temperature, were used to insure a closed system at the gas inlet and outlet ends of the apparatus.
The reactor was turned at 28 rpm. The reactor temperature was monitored by means of an Inconel-protected thermocouple which passed through the driven trunnion into the center of the reactor. A cleanout rod, introduced through the outlet seal, was provided to remove any plug of ferric chloride which formed at the entrance to the sublimate receiver. The outlet tube of the reactor was provided with an internal glass-wool filter which prevented the solid material of the charge from being carried into the receiver with the sublimate. The apparatus is shown in use in figure 2.
Glass flowmeter tubes were used for metering the gases, a manometer for indicating positive or negative pressure in the system, and a trap and caustic scrubber for regulating and containing the exit gases of the system. The chlorine supply cylinder was mounted on a scale, which permitted measurement, by difference, of the amount of chlorine used at any instant.
Preparation of Charge
Chlorination tests performed with the rotary chlorinator indicated that minus 100-mesh particles would chlorinate rapidly and efficiently. The
high-carbon ferrochromium produced from the Mouat chromite was brittle and was easily reduced to minus 100-mesh particles. The low-carbon ferrochromium was somewhat ductile and was more difficult to reduce to the desired minus 100-mesh size. Coarser particles developed a coating of chromium chloride that prevented complete penetration of the particle by the chlorine gas and left a portion of the ferrochromium unchlorinated.
Chlorination Procedure
A 100-gram charge of minus 100-mesh ferrochromium was placed in the reactor drum with a few Pyrex beads to help keep the charge loose. After the glass-wool filters were placed in the inlet and outlet trunnions, the apparatus was assembled and tested for gas leaks. A small flow of helium gas was passed through the system while the furnace was brought to operating temperature to remove any moisture in the charge and apparatus, and the drum was rotated to prevent packing of the charge. When the drum temperature reached 340° to 400° C, the helium flow was shut off and the chlorine flow started.
Once the reaction commenced, the chlorine-flow rate and the current tothe heating furnace were regulated to prevent the exothermic reaction from raising the temperature above 525° C; and, in general, the temperature of the chlorination was held between 340° and 525° C. The manometer showed a negative pressure when the chlorine flow was less than the absorption rate and a positive pressure when the chlorine flow exceeded the absorption rate or the outlet became plugged with sublimate. Periodic use of the cleanout rod kept the outlet open. As the reaction subsided, the gas flow was reduced to prevent loss of chlorine. The chlorination was complete when the charge ceased to absorb chlorine. The system was then cooled to room temperature under a helium atmosphere.
Leaching Procedure
The product in the reactor consisted principally of chromium chlorides, small amounts of iron chlorides, free carbon, and unreacted ferrochromium. It was free flowing and could be poured from the reactor. Any adhering material was washed from the reactor with water. The soluble portion of the product was dissolved in water. Dissolution proceeded rapidly if there was sufficient CrCl2 in the product to catalyze the solution of the CrCl3. When the chromium product was entirely CrCl3, it was necessary to slurry the product with water, add amonium hydroxide (NH4OH) to decompose the CrCl3, and acidify the slurry with hydrochloric acid (HCl) to dissolve the insoluble hydroxides. Any remaining insolubles were removed by filtration, The insoluble residue was then dried, weighed, and analyzed. The filtrates and washings were combined and diluted to 1 liter for analysis.
Experimental Results
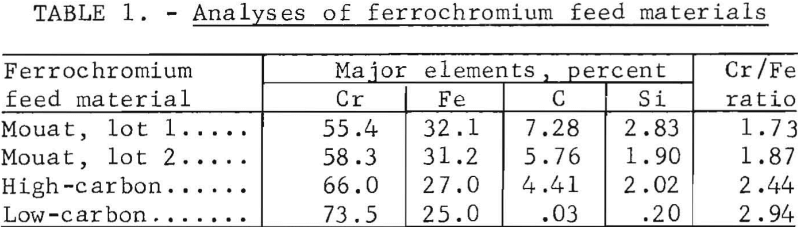
Three types of ferrochromium materials were investigated. The majority of tests were made using a high-carbon ferrochromium which had been prepared by electric arc smelting of Mouat chromite concentrates. The two lots of Mouat ferrochromium tested varied slightly in composition. A standard high-carbon-ferrochromium sample, from the National Bureau of Standards, and a commercial low-carbon-ferrochromium sample were also investigated, using the experimental procedures developed for the chlorination of the Mouat ferrochromium. The major elements contained in the ferrochromium feed materials are given in table 1.
A mixture of equal volumes of chlorine and helium was used in the chlorination of Mouat ferrochromium, lot 1 ; the helium was to serve as a carrier gas to help remove the FeCl3 vapor from the reactor. However, subsequently tests on lot 2 with chlorine alone proved that helium not only was unnecessary for removal of the FeCl3 but also wasted chlorine by sweeping unreacted chlorine from the reactor.
The chlorination products were mainly FeCl3 and CrCl3. A small amount of CrCl2 was produced in some of the tests. Most of the FeCl3 was sublimed out of the reactor and was collected in the glass receiver. The FeCl3 sublimate was light and fluffy; tumbling in the rotating glass receiver helped compact it. Recovery of the FeCl3 product was not quantitative due to the mechanical limitations of the apparatus . The reactor products removed on conclusion of the tests were CrCl3, small amounts of CrCl2 and FeCl3, free carbon, and unreacted feed material.
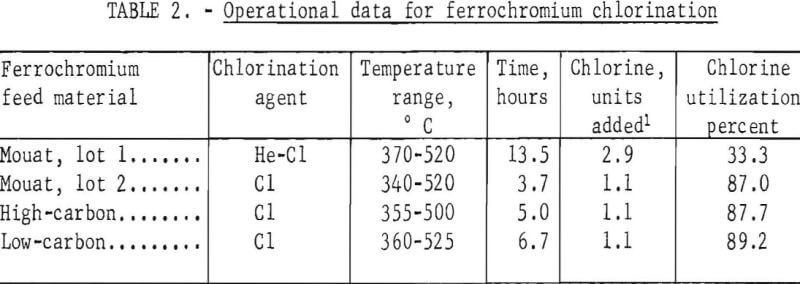
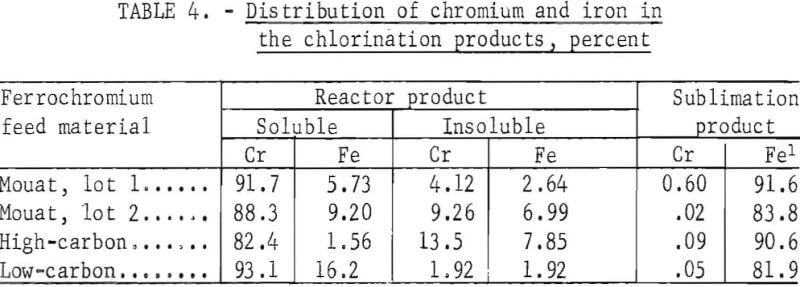
Results from a typical run with each type of feed material appear in tables 2-4. The operating conditions used for chlorination of the ferrochromium are shown in table 2. Analyses of the products are given in table 3, and the chromium and iron distributions in the chlorination products are shown in table 4.
The sublimation products were in all cases essentially FeCl3. The higher chromium value of 0.37 percent in the sublimation product of Mouat, lot 1, was believed to be due to mechanical carryover with the helium. Very little chromium was found in the sublimation products of the other feed materials.
Leaching of the reactor product recovered 82.4 to 93.1 percent of the chromium content of the ferrochromium in the leach solution. The efficiency of the reaction was shown by the small amount of chromium and iron left in the insoluble residue. An average of 89 percent of the chromium and 95 per¬cent of the iron contents of the ferrochromium feed materials were converted into chlorides by low-temperature chlorination.
There was no major difference in the chlorination characteristics of the three types of ferrochromium investigated. The amount of iron removed by sublimation varied from 81.9 to 91.6 percent. This variation was probably due more to physical variations in the reaction apparatus, such as permeability of the glass-wool-filter plugs and operating techniques, than to differences in the chlorination characteristics of the different types of ferrochromium.
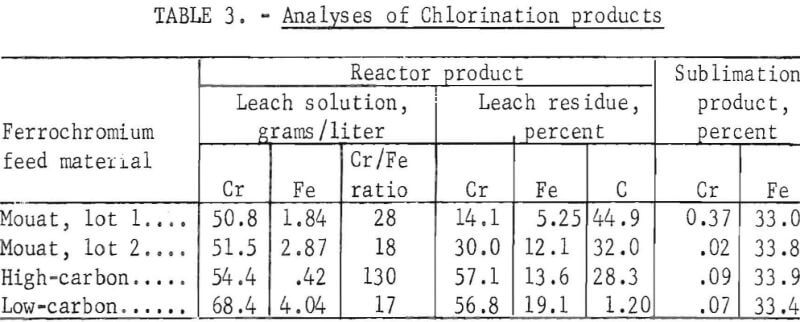
X-ray diffraction analyses on the Mouat ferrochromium and the high-carbon ferrochromium showed that Cr7C3 was a major constituent of each; Cr23C6 was found to be a minor constituent of the Mouat ferrochromium. Chlorination of the chromium carbides liberated the carbon as free carbon in all chlorination tests. The carbon content of the ferrochromium feed materials was recovered, primarily as free carbon, in the leach residue.
This chlorination process may be useful in upgrading the chromium content of domestic high-carbon ferrochromium by removing the majority of the iron as a volatile chloride. The bulk of the chromium is converted to a soluble chloride which remains in the reactor, This chromium product could be reduced to chromium metal by aqueous or molten salt electrolyses or could be converted into other chromium chemicals.
