Table of Contents
To increase metal recoveries and to minimize pollution by improved extraction technology, the Bureau of Mines investigated a hydrometallurgical technique to recover copper, lead, nickel, and cobalt from lead smelter mattes. The metals were converted from insoluble sulfides to soluble chlorides using a HCl-Cl2-O2 leaching system. Parameters studied in a 50-gallon, glass-lined reactor were the ratio of hydrochloric acid to chlorine, chlorine consumption per pound of matte, oxygen reaction pressure, and temperature. The matte material used contained, in percent, 19.7 Cu, 52 Pb, 2.9 Ni, 1.5 Fe, 0.38 Co, 0.19 As, 0.41 Zn, and 0.12 Cd. Extraction of Cu, Pb, Ni, Co, and Cd ranged from 92 to 98 pct, with concomitant extractions of Fe and As of <0.1 pct. The Cu and Pb were recovered as electrowon metals, while the Ni, Co, and Cd were recovered as mixed hydroxides.
To help insure an adequate supply of minerals and metals to meet U.S. economic and strategic needs with minimal degradation to the environment, the Bureau of Mines investigated the recovery of metals from complex sulfide ores, concentrates, and waste materials. This work is part of a research program aimed at advancing hydrometallurgical technology.
Sulfide matte from commercial lead smelters is a material containing Cu, Pb, Ni, Zn, Co, and Cd that is currently being discarded or not fully used. The matte materials are difficult to process because of stringent emission standards for arsenic and lead and are currently being stockpiled or exported.
In previous research, the Bureau of Mines investigated recovery of metal values from a variety of complex metal sulfide ores and concentrates using a chlorine-oxygen or ferrous chloride-oxygen pressure leaching system. These systems involve leaching the solids in a slurry using different sources of chloride—such as chlorine gas, hydrochloric acid, or ferrous chloride—and subsequently reacting the resulting slurry with oxygen at less than 50 psig pressure and a temperature of about 110° C. Metals such as Cu, Ni, Zn, Co, and Cd were converted to soluble metal chlorides; Fe, As, and Sb were converted to insoluble oxides, and Pb was converted to insoluble chloride. Most of the sulfide sulfur was converted to elemental sulfur, which remained in the residue.
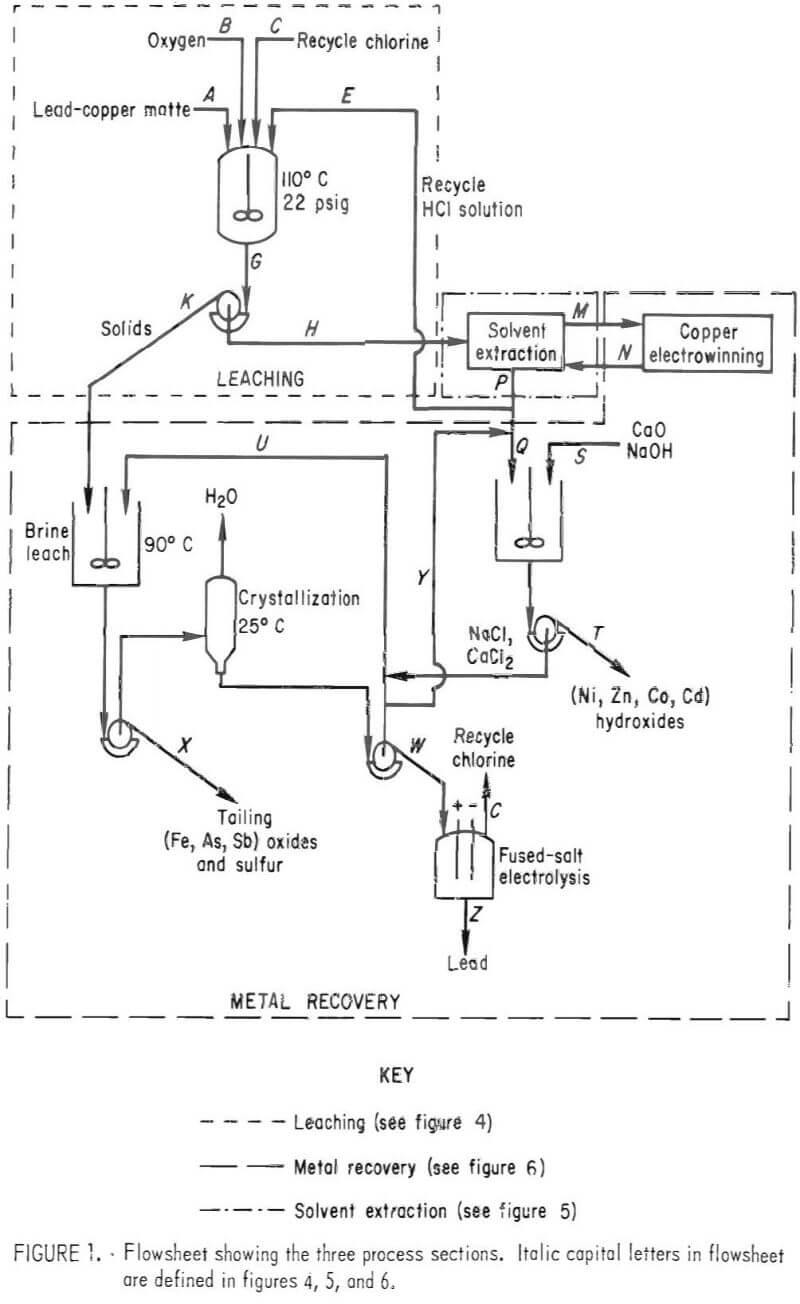
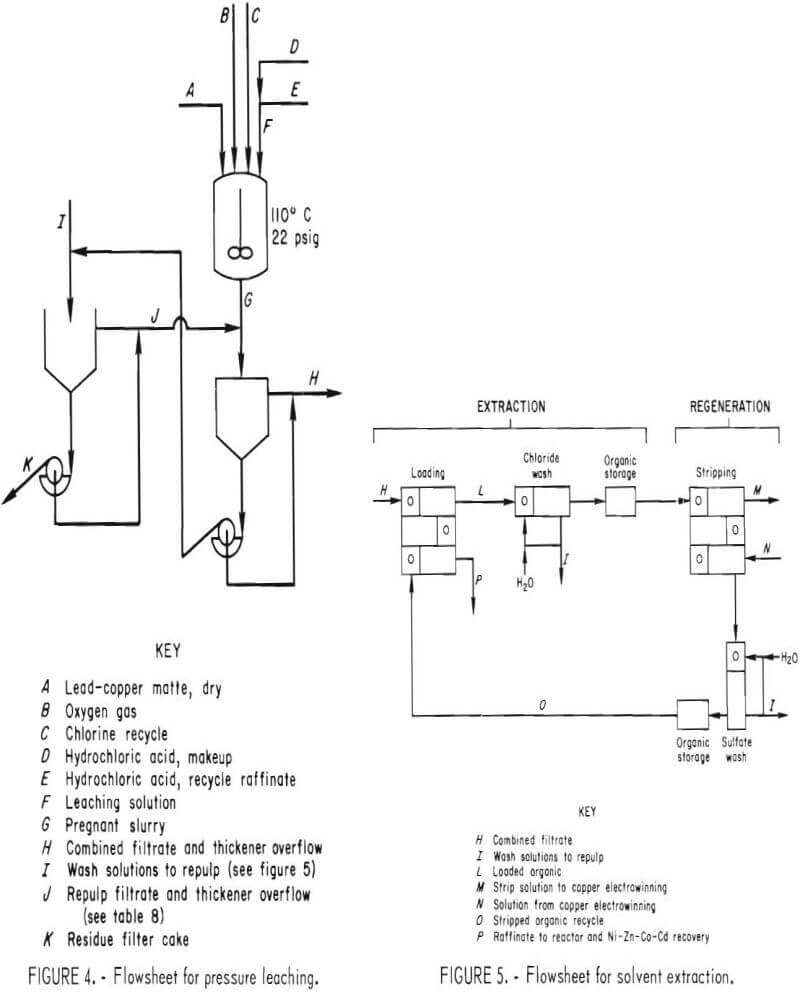
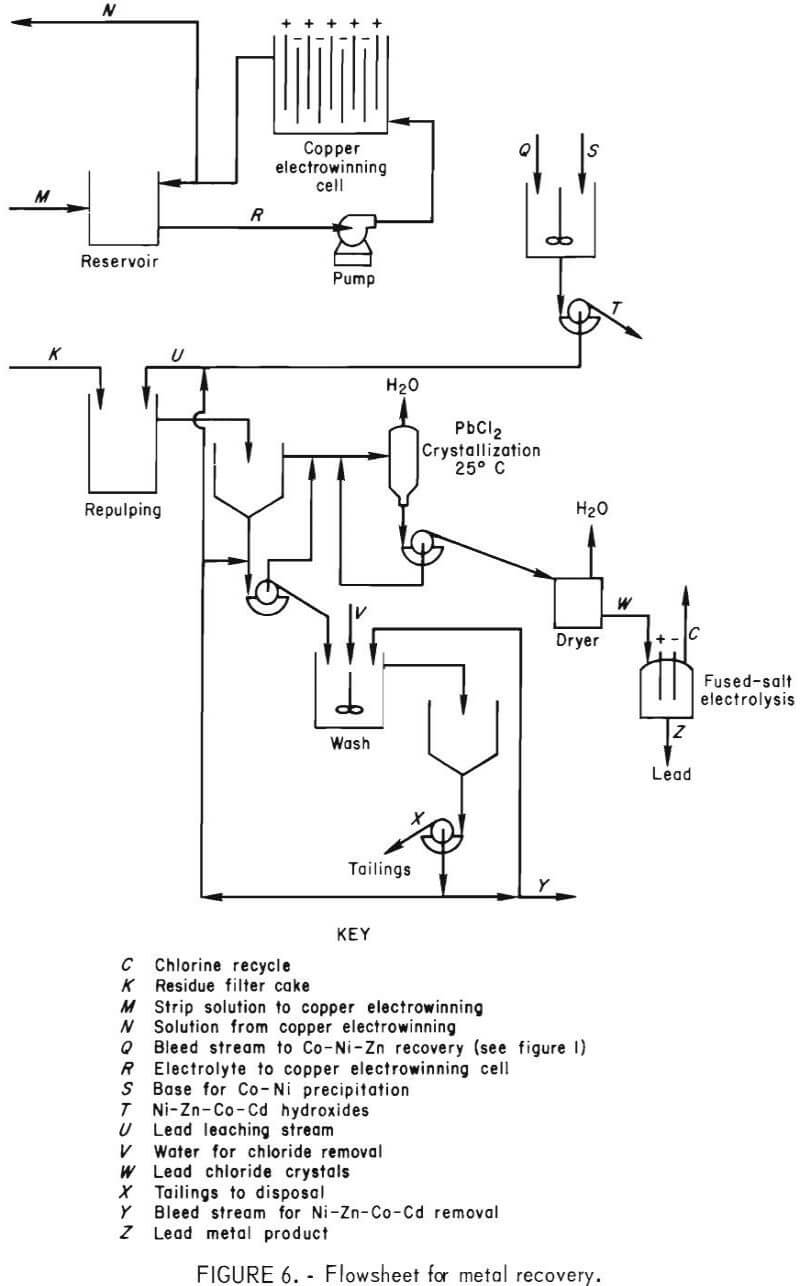
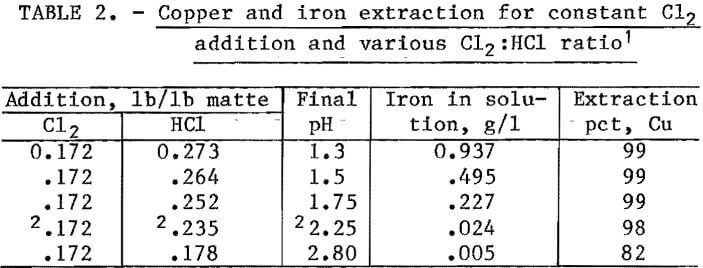
This report describes the application of the chlorine-oxygen leaching system to a complex lead sulfide (PbS) matte and outlines a possible route for the recovery of Cu, Pb, Ni, Zn, Co, and Cd from a waste material. The data presented define the operating parameters investigated and their effects on extraction and purity of products. The flowsheet investigated, shown in figure 1, can be divided into three sections: (1) leaching, (2) solvent extraction, and (3) metal recovery. These sections will be discussed in detail.
Materials and Equipment
Feed Preparation
An 11,000-lb sample of minus ¼-inch PbS matte was received from a smelter in Buick, Mo. Screening tests performed at the smelter indicated that the lead content of the plus 20-mesh fraction was suitable for recycle to the smelter. Therefore, the matte was screened, and the minus 20-mesh material was leached. A clean and consistent feed, which did not abrade the leaching reactor, was obtained. The head assays of the screened fractions from the matte sample are presented in table 1.
Leaching
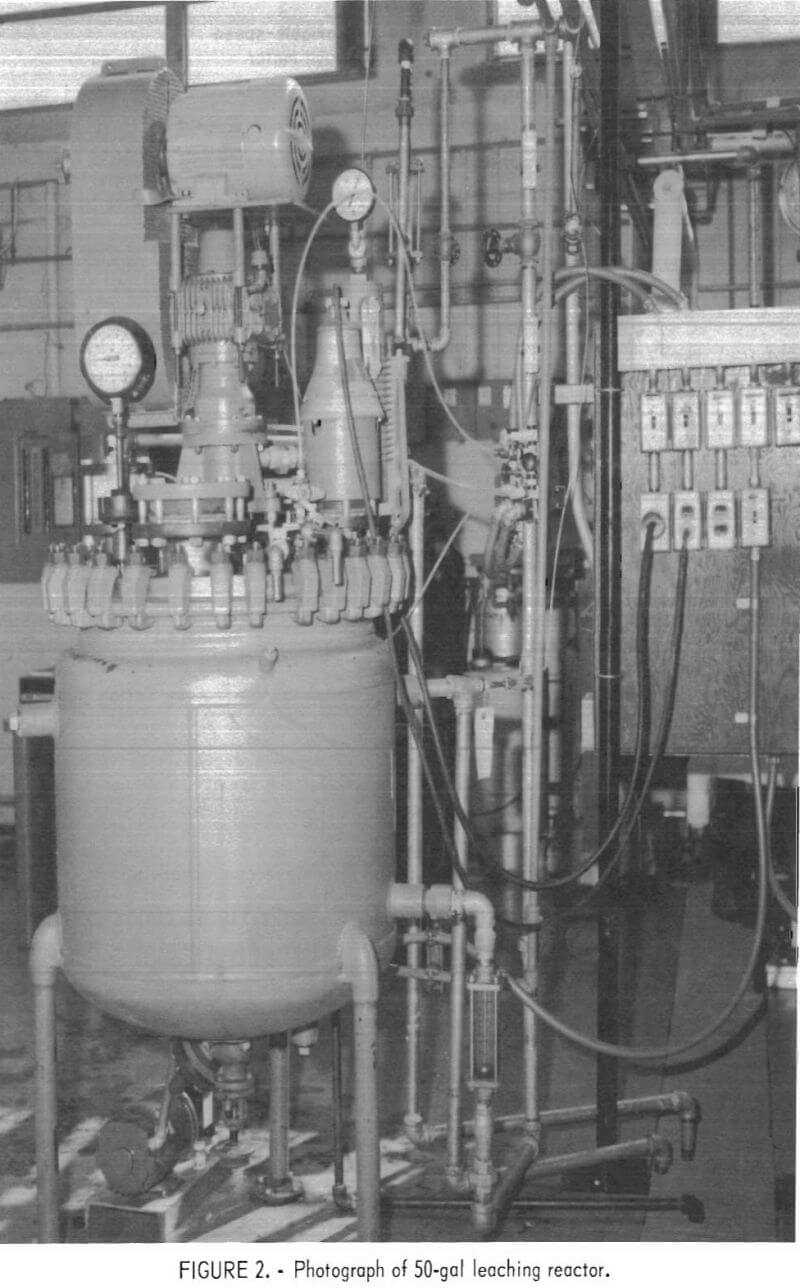
The leaching reactor, shown in figure 2, was a 50-gal, series P, glass-lined, jacketed Pfaudler reactor with a Bureau-designed titanium lid and agitation system. A cutaway view of the reactor is shown in figure 3. The impeller-baffle-draft tube assembly was designed to increase the reaction rate by increasing the surface area of the gas-slurry interface through improved gas dispersion and slurry mixing. An adjustable slot was provided in the draft tube to regulate the flow of slurry and insure gas-slurry contact at the impeller. The impeller speed was adjusted to draw the gas-slurry mixture down the draft tube and obtain vigorous agitation. Typically, the impeller rotated at 400 rpm with a tip speed of 840 ft/min.
The liquid-solid separation equipment (fig. 4) consisted of two 3-ft-diam by 28-in.-deep, rubber-lined thickeners, a 9-ft² rubber-coated scraper discharge rotary vacuum filter, and associated fiber-reinforced plastic (FRP) tanks.
Solvent Extraction
The solvent extraction circuit (fig. 5) consisted of three extraction stages, a chloride removal water wash stage, three acid strip stages, and a sulfate removal water wash stage. Each stage used a mixer-settler unit, 7.8 in. wide by 38.5 in. long by 12 in. deep. The mixing chamber was 7.8 by 7.8 by 12 in. deep. The volumes of the mixing and settling chambers were 10 and 40 liters, respectively. The mixers were pumping-type impellers connected to variable-speed drives. Forty-five-liter reservoir tanks were inserted in the circuit before and after the stripping section to allow the wash water to disengage from the loaded organic and to decrease sulfate carryover to the process solution.
The solvent extraction units used were commercially available FRP and locally built acrylic plastic mixer-settler units. The associated reservior tanks were FRP and polyethylene.
Metal Recovery
A copper electrowinning cell (fig. 6), 12 in. wide by 18 in. long and 10 in. deep, was constructed using acrylic plastic. The nine 6-pct antimonial lead anodes and eight copper starter sheets were 8 by 11.5 in. and spaced 1 in. apart. A 50-liter reservior was used between the final stripping stage of solvent extraction and the electrowinning cell. This permitted a higher flow rate through the cell than that from the stripping stage and minimized anode corrosion at the current densities used. A controlled-voltage selenium rectifier supplied the dc power.
Cobalt and nickel were precipitated from the raffinate bleedstream in a 15-gal polyethylene stirred tank. The precipitation pH was controlled by a pH monitor, which regulated the caustic addition from a vibrating feeder. The precipitate was recovered in a laboratory filter.
Lead chloride (PbCl2) crystals from the brine leaching circuit were dried, and the lead was electrowon in a laboratory-scale fused-salt cell. Extensive research on fused-salt electrolysis of PbCl2 has been reported by the Bureau in previous publications.
Procedures
The data presented were collected from batch experiments. The conditions were controlled to simulate the flowsheet shown in figure 1. Streams are identified for reference in other figures or tables.
Leaching
The 50-gal reactor was charged with either fresh HCl solution or recycle solution from the solvent extraction circuit and makeup acid required for Cu, Ni, Zn, Co, and Cd dissolution and Pb conversion. In order to produce a filtrate with approximately 30 g/l Cu for feed to the solvent extraction circuit, 34 lb of lead matte were added to 102 liters of acid solution at ambient temperature. The reactor was closed and the selected amount of chlorine gas (Cl2), between 5.0 and 6.2 lb, was added at ambient pressure. Approximately 4 min was required for the Cl2 addition at a flow rate of 1.5 lb/min. Since the reaction was exothermic, the slurry temperature increased to approximately 90° C during chlorine addition.
The HCl/Cl2 ratio for maximum metal extraction was determined in a series of tests using a constant amount of Cl2 and varying amounts of HCl. The total Cl2 and HCl additions were based on the metal content of the matte and on theoretical reagent consumption calculated according to equations 1 and 2:
PbS + Cl2 → PbCl2 + S°………………………………………………………………(1)
MS + 2HCl + ½ O2 → MCl2 + H2O + S°………………………………………(2)
where MS represented metal sulfides, such as Cu, Ni, Co, Zn, and Cd sulfides.
After Cl2 addition, the reactor was pressurized with oxygen (O2). The pressure was maintained until the O2 consumption rate decreased to less than 0.1 scfm. The majority of the tests were performed at 22 psig. Temperature was maintained by using steam or water in the jacket of the reactor. A number of tests were performed to determine the effects of temperature and pressure on leaching efficiency and reaction time. During the operation of the process demonstration unit (PDU), 1,750 lb of matte was treated in the leaching section.
Solvent Extraction
Filtrate recovered from liquid-solid separation of the reactor slurry was treated by solvent extraction to (1) separate Cu from Ni, Zn, Co, and Cd, (2) transfer the copper in the chloride leaching solution to the sulfate stripping solution, (3) provide a concentrated copper sulfate feed to the electrowinning circuit, and (4) produce HCl for recycle to the reactor. Equations 3 and 4 show the reactions that occur, where M represents Ni, Zn, Co, or Cd.
2R-H(org) + CuCl2(aq) + MCl2(aq) → R2Cu(org) + 2HCl(aq) + MCl2(aq)……………….(3)
R2Cu(org) + H2SO4(aq) → 2R-H(org) + CuSO4(aq)………………………………………………(4)
Figure 5 shows the flowsheet used in the solvent extraction section.
The extractant had to be specific for copper at a pH of less than 1, be strippable with a H2SO4 solution, be acceptable to electrowinning, and have adequate transfer capacity to allow a reasonable organic-to-aqueous ratio (O:A).
The organic extractant, LIX 70, was selected after a literature search (2) and laboratory tests to confirm its transfer capacity and selectivity. To provide a transfer capacity of approximately 3.0 g/l, 30 vol-pct. LIX 70 diluted with Socal 355 solvent and a stripping solution of 220 to 240 g/l of H2SO4 were used.
The pH of the feed to the solvent extraction section ranged from 1.3 to 2.7 (1.83 to 0.073 g/l HCl). The Cu2+ in the feed was 22 to 31 g/l. The raffinate contained 24 to 49 g/l HCl and 0.8 to 2.1 g/l of Cu2+. The O:A ratio was varied from 6:1 to 9:1 to accommodate a variation in copper concentration in each batch of feed and to maintain the raffinate at less than 5 g/l Cu.
The total flow ranged between 1.45 to 1.65 l/min. The raffinate, which contained high concentrations of acid, Ni, Zn, Co, and Cd and a small amount of Cu, was recycled to the leaching system. Fresh HCl or H2O was added to maintain a consistent feed. After the Ni, Zn, Co, and Cd had built up to approximately 7.0, 2.2, 1.7, and 0.55 g/l, respectively, a bleedstream for metal recovery was taken.
Loaded organic at an O:A of 2:1 was washed with water to decrease the chloride carryover by entrainment and then stripped with recycled electrolyte from the copper electrowinning cell that contained 17 to 27 g/l Cu. The organic transfer capacity was 2.8 g/l Cu. Typically, the organic was loaded to 7.9 g/l Cu and stripped to 5.1 g/l Cu. The spent electrolyte concentration was typically between 220 to 240 grams of H2SO4 and was maintained in this range by adding concentrated acid, which insured consistent stripping of the loaded organic. Stripped organic was contacted with water to minimize sulfate transfer to the raffinate during the loading step.
Metal Recovery
A detailed flowsheet for the metal recovery section is shown in figure 6. Copper was recovered in an electrowinning cell that simulated a standard tank-house producing cathode copper and regenerated H2SO4. The current density on the electrodes was approximately 15 amp/ft². A current efficiency of approximately 90 pct was obtained. The copper in the electrolyte was maintained at about 35 g/l. Anode corrosion was expected if the flow through the electrowinning cell was the same as that of the stripping acid from the solvent extraction section. Therefore, the 0.6 to 0.75 l/min flow from stripping was introduced near the middle of a 50-liter reservoir, and a 6- to 7-l/min stream was pumped from the bottom of the reservoir up through the electrowinning cell. A bleedstream to maintain the stripping acid volume was taken from the spent electrolyte returning to the reservoir.
Nickel, zinc, cobalt, and cadmium contained in the bleedstream from the raffinate returning to the leaching circuit were precipitated as hydroxides by increasing the pH to 10 with caustic soda. The precipitate was repulped 50:50 in water, filtered, and dried.
Lead chloride was extracted by repulping and washing the reactor discharge filter cake with 80° to 90° C solution containing 240 g/l NaCl. A 300-lb batch of filter cake was repulped in hot brine solution. The undissolved solids were allowed to settle. The supernatant was removed and cooled to precipitate PbCl2. The PbCl2 crystals were filtered from the supernatant, and the filtrate was recycled within the system. The above procedure was continued until the brine solution, after contact with the solid, contained nearly the same concentration of lead as before contact. Hot water was used to leach the remaining lead and wash out the NaCl. Leachable lead was monitored by taking a 200-gram sample of filter cake from each of the leaching tests. They were treated in the laboratory by repeated repulping with fresh, hot brine solution. These small samples were used to monitor lead extraction under different leaching conditions without handling the entire reactor charge. The results of the 300-lb leaching test were consistent with those obtained in the 200-gram tests.
Fused-salt electrolysis of PbCl2 in a NaCl-LiCl2 bath is a technique pioneered by the Bureau. The technique consists of heating the salt in a eutectic bath to fluidity, then passing dc current between two graphite electrodes so that the PbCl2 is converted to lead and chlorine, which is recycled to the leaching system.
Results and Discussion
Leaching
In the initial pilot demonstration unit (PDU) leaching experiments, the amount of Cl2 (pounds per pound of matte) was kept constant and the amount of HCl added was varied. The results are summarized in table 2. As the HCl was increased from 0.178 to 0.252 lb/lb of matte, the copper extraction increased from 82 to 99 pct and the concentration of iron in solution increased from 0.005 to 0.227 g/l. The best leaching conditions were 0.235 lb of HCl and 0.172 lb Cl2 per pound of matte, followed by the addition of oxygen to the slurry at 90° to 106° C with an overpressure of 48 psig. The total reaction time for the PDU apparatus was 1 hour because the slurry had to be heated to increase the oxygen reaction rate. Copper extraction was 98 pct, and only 0.024 g/l Fe was dissolved.
The effect of varying the amount of Cl2 added was also investigated. Total chloride ions per pound (Cl-/lb) matte was maintained at the best leaching conditions. By varying the ratio of chlorine to HCl, the Cl-/lb of matte was held constant, but the oxidation was altered according to equations 5 and 6:
Cl2 + H2O → OCl- + 2H+ + Cl-…………………………………………………..(5)
HCl ↔ H+ + Cl-…………………………………………………………………………(6)
Table 3 summarizes the test results. The data indicate that the amount of Cl2 can be varied, but enough HCl must be added to satify the Cl- requirements of equations 1 and 2. The oxygen required was constant at 0.064 lb O2/lb matte; however, the rate of 02 addition varied with temperature, pressure, and available reactants. The effects of pressure at constant temperature, and temperature at constant pressure, were studied by measuring the time required for the O2 flow to decrease to 0.1 scfm. The results are summarized in tables 4 and 5. The data show that the rate of reaction is both temperature and pressure dependent for the range of conditions investigated. If the pressure was increased from 22 to 48 psig at controlled temperature (average 105° C), the reaction time decreased by 38 pct, from 32 to 20 min. When the pressure was maintained constant at 22 psig, an increase in the temperature from 80° to 113° C decreased the reaction time by 60 pct, from 52 to 21 min.
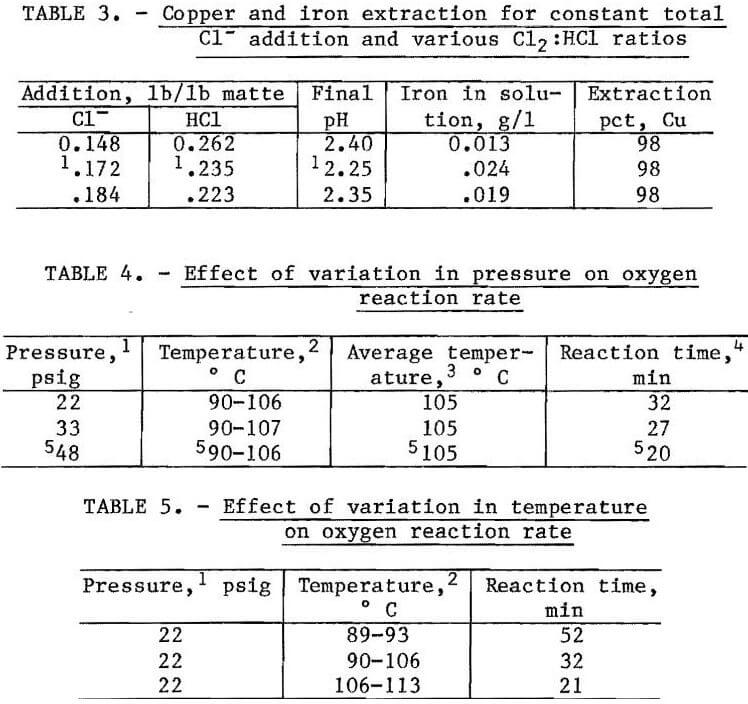
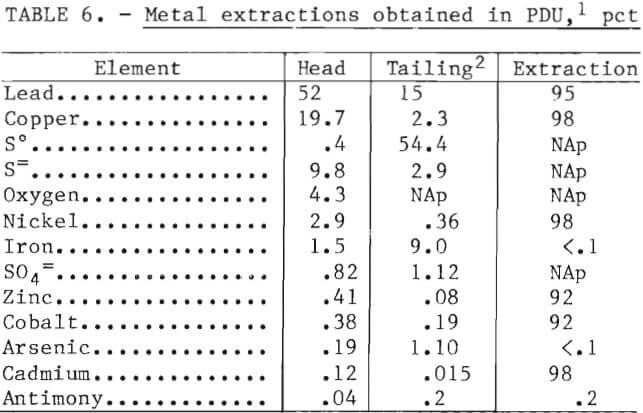
Table 6 summarizes typical metal extractions obtained at the best operating conditions (see tables 3 and 4). Extraction of Cu, Pb, Ni, Co, Zn, and Cd ranged between 92 and 98 pct, while 0.1 to 0.2 pct of the Fe, As, and Sb were solubilized.
Liquid-Solid Separation
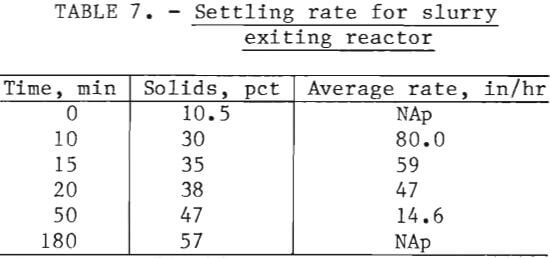
The slurry from the reactor had a specific gravity of 1.1 and contained 10.5 pct solids. The suspended solids in the slurry settled rapidly when 0.05 lb of Separan MGL flocculant per ton of solids was used. The resulting solutions were clear. In table 7, the settling rate and solids content of the settled material are given as a function of time. Approximately 1 hour retention time in the thickeners was required.
The filtration rate of the 57 pct solids slurry (0.1 ft² test leaf) was 225 to 250 lb/ft²/hr. Moisture content of the cake was 17 pct.
The liquid-solid separation and the solution flow sequence used in the PDU are shown in figure 4. The slurry from the reactor was settled in a thickener and then filtered on a drum filter. The filter cake was repulped with a bleedstream taken from the chloride wash solution in the solvent extraction circuit. The resulting slurry was settled and filtered.
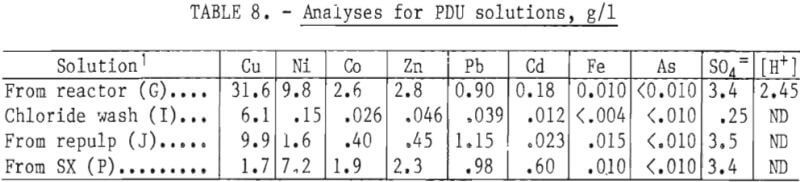
Typical solution analyses at the best extraction conditions in the PDU (tables 3 and 4) are shown in table 8.
Solvent Extraction
The combined process solutions from both thickeners and filters assayed (in grams per liter): 22 to 31 Cu, 2.9 to 13 Ni, 1.0 to 2.8 Co, 0.9 to 2.0 Zn, 0.04 to 0.19 Cd, and 0.01 to 0.09 Fe. The extraction section of the circuit was operated at an O:A flow ratio of from 6:1 to 9:1, depending on the copper concentration in the feed. With an aqueous flow rate of 0.15 to 0.25 l/min, a corresponding organic flow rate of 1.2 to 1.5 l/min was required. If the total flow was between 1.45 and 1.65 l/min, good disengagement was obtained. The water washes of the loaded organic and of the stripping section were operated at an O:A flow ratio of 2 to 1 or an aqueous flow of 0.60 to 0.75 l/min.
During the PDU operation, 2,270 liters of aqueous process solution was treated. The minimum flow of the organic at an O:A ratio of 6:1 was 13,620 liters. Since the organic circuit capacity was 253 liters, the organic was recycled approximately 53 times during operation of the solvent extraction circuit. No change in the transfer capacity or loading of the organic phase was noted.
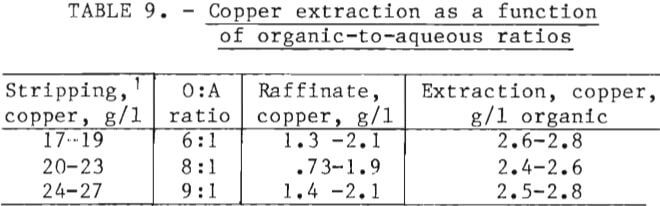
Copper extraction from aqueous chloride process solution during 200 hours of continuous operation of the solvent extraction circuit is summarized in table 9. Copper transfers of 2.4 to 2.8 g/l of organic were obtained. The transfer was 80 to 90 pct of theoretical and provided a raffinate low in copper and an organic capable of handling any surge in the copper load.
Metal Recovery
During 200 hours of electrowinning the chloride ion built up from 0.19 to 0.42 g/l, while Fe, Zn, and Co concentration remained the same and the nickel concentration increased from 0.010 to 0.130 g/l, as shown in table 10. Cadmium concentration was not monitored. The chloride that entered the electrolyte was entrained wash water in the loaded organic that had insufficient time to disengage because only 30 to 37.5 min of disentrainment time was possible. The time required for complete disengagement of entrained water was at least 2 hours. To operate the circuit successfully over long periods of time, a storage tank for the loaded organic must have a capacity for at least 2 hours of retention.
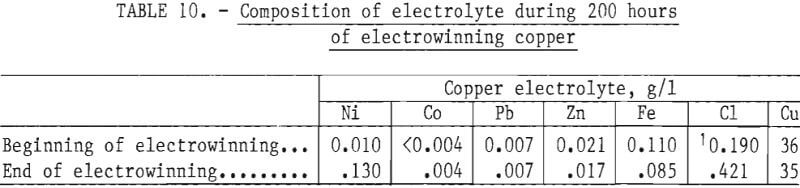
The copper deposited during electrowinning was dense and cohesive, and had a purity of 99.95 pct. Lead was the major contaminant. Anode corrosion for the test period was negligible.
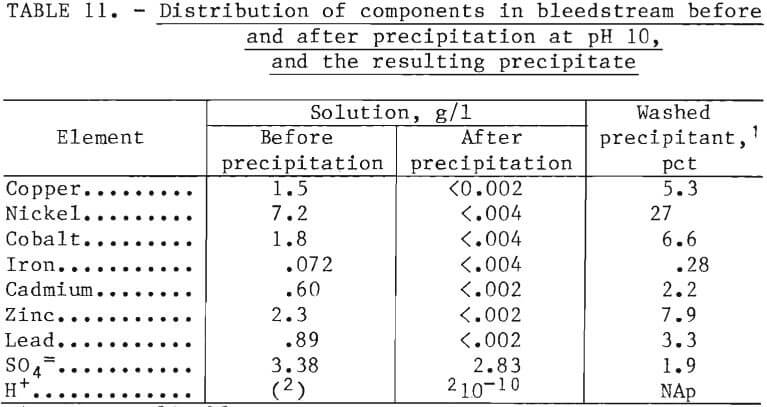
Nickel, zinc, cobalt, and cadmium were recovered by taking a bleedstream (0.02 l/min) from the solvent extraction raffinate stream and precipitating all the metals by increasing the pH to 10.0 with caustic. Results obtained in the PDU are shown in table 11. After precipitation, all metal ions were below detectable limits in the filtrate. The metal hydroxide filtered slowly and had a high moisture content. The precipitate was repulped to 50 pct solids, filtered, and dried.
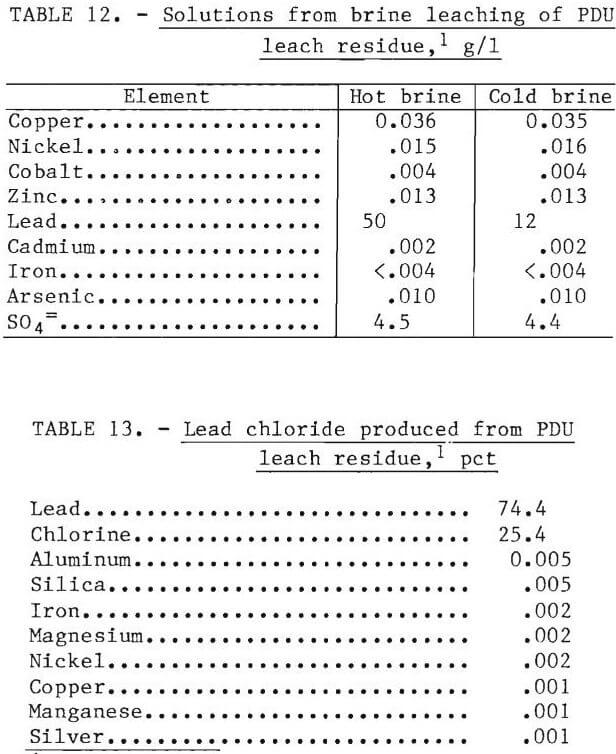
Lead was recovered from the leaching residue by hot brine leaching-crystallization and fused-salt electrolysis of the lead chloride. The Bureau of Mines has conducted considerable research on recovery of lead from galena concentrates. Since the residue produced in the PDU was similar to residue previously investigated, only 300 lb of residue was leached with brine. The average head assay was 52 pct Pb. Lead extraction was 95 pct. The solution analyses show that less than 50 ppm (each) Cu, Ni, Co, Zn, Cd, Fe, and As were dissolved during brine leaching at 85° to 95° C. Crystallizing PbCl2 by cooling the hot brine to room temperature decreased the lead concentration from 50 to 12 g/l, while the concentration of the other metals remained essentially constant. Analyses of the brine solutions and of the PbCl2 produced are shown in tables 12 and 13. Lead metal of more than 99.99 pct purity was produced in a 1,000-ml fused-salt electrolysis cell.
Recovery of Metal in Lead Smelter Matte by Chlorine Oxygen Leaching
Metal values were recovered from a lead smelter matte using a chlorine-oxygen process to solubilize the Cu, Ni, Zn, Co, and Cd and convert the lead to a chloride. A subsequent hot brine leach was used to extract the lead from the residue. The copper was separated from the Ni, Zn, Co, and Cd by a solvent extraction technique; this demonstrated the feasibility of leaching in a chloride system and electrowinning in a sulfate system. Extractions of 98 Cu, 98 Cd, 96 Pb, 97 Ni, 93 Co, and 92 Zn (in percent) were achieved. The electrowon copper and lead were of 99.95 and 99.99 pct purity, respectively.
The processing proposed in the flowsheet will produce high metal recoveries and no sulfur emissions or discharge of acid solutions. This is an alternative to the stockpiling or exporting of metal resources.
