Table of Contents
Careful studies of the fundamentals of chromite flotation have been made recently in the United States and in Poland. Palmer (1972), Palmer, Fuerstenau and Aplan (1975) and Fuerstenau and Palmer (1976) have demonstrated the importance of the presence or absence of charged metal hydroxy species in such flotation. These species appear particularly to influence anionic flotation of chromite when the mineral is being floated above its point of zero charge (PZC). These researchers considered oleate, saturated carboxylic acids and alkyl sulfates as collectors for the mineral. Aqueous metal species studied included Al(III), Cr(III), Fe(II), and Mg(II).
The work of Sobieraj and Laskowski (1973) considered in detail the effect of Al(III) on chromite flotation. Collectors studied included laurate, dodecylamine and several amphoteric surfactants. Both natural chromites and synthetic spinels were studied. Sobieraj, Haber and Laskowski (1972) also studied the mechanism of surface oxidation of chromite and its effect on surface characteristics and flotation behavior of the natural chromites and the synthetic spinels.
The present work reports on the effects of various pretreatments of chromite on the mineral’s flotation behavior when sodium dodecylsulfate (SDS) and dodecylammonium chloride (DAC) were used as collectors. In addition, for comparison with flotation data, the electrophoretic mobility of chromite was measured in selected environments and adsorption of SDS onto chromite as a function of pH was studied. Also, dissolution of several metals from chromite placed in acid solution was monitored.
Materials
The chromite studied was from the Stillwater Complex, Montana and was specifically a table concentrate supplied by the Salt Lake City Metallurgy Research Center, United States Bureau of Mines. An analysis of the material is shown in Table I.

Chemicals used in the experiments included high purity sodium dodecyl-sulfate (SDS) and dodecylammonium chloride (DAC) and reagent grade HCl, reagent grade Na2CrO4 and carbonate free, reagent grade NaOH. In the methylene blue analytical procedure used with the adsorption experiments high purity methylene blue and chloroform were used.
For the flotation experiments the mineral was ground in a pebble mill and the 100/150 mesh fraction used for flotation. A 150/325 mesh fraction was used for the adsorption experiments. For the electrophoresis work a minus 325 mesh fraction of the chromite was used.
The water used was double distilled, first in a tin lined still and finally in a glass still.
Flotation
The flotation tests were carried out in a modified Halimond tube. Details of use of this apparatus have been described elsewhere (Fuerstenau, Metzger and Seele, 1957, and Smith and Lai, 1966). Conditioning time in collector solution prior to flotation was six minutes. Before this conditioning time the mineral samples were pretreated in various different ways as subsequently noted. Flotation time was one minute. Purified nitrogen, the flotation gas, was passed through the cell at the rate of one tenth liter per minute. Temperature during the tests was 22-26°C.
Microelectrophoresis procedure
The electrophoretic mobility measurements were, performed using a commercially available microelectrophoresis apparatus, the Zeta Meter. Details on the construction and use of this device can be found elsewhere (Riddick, 1968, and Smith and Trivedi, 1974). In the present work the -325 mesh mineral samples were pretreated by aging in water for varying lengths of time as noted subsequently in the text. To aid this procedure an automatic transfer pump, accessory to the Zeta Meter, was employed. Temperature ranged between 22-26°C.
Adsorption tests
The adsorption tests were carried out in 100 ml beakers. 0.2 g of the chromite samples were placed in the beaker and 30 ml of 5 x 10 -5 M SDS solution were then added. The pH was adjusted using small quantities of either NaOH or HCl to raise or lower pH. A blank solution of SDS at the same pH was also prepared for comparison. The beakers were placed in an aquatherm bath shaker (temp. 25°C) and agitated for 2 hrs. After agitation and a short settling period pH was measured (the reported pH value) and a 25 ml aliquot of the SDS solution withdrawn for analysis. In some cases it was necessary to centrifuge the sample. The analytical procedure used for SDS analysis was the methylene blue method of Jones (1945).
Laboratory Experiment
Figure 1 shows the electorphoretic mobility (EM) of the chromite studied as a function of pH and aging time in water. The mineral was fresh ground before the tests. Note that the point of zero charge (PZC) of the mineral is about pH 5.2 at short aging and that it shifts to more acid values after 2 hr and 6 hr aging in water. Also shown on the figure is a curve illustrating the effect of 1 x 10 -4 M SDS on the EM of the mineral (aging time in water <½ hr). In general the presence of SDS causes the electrophoretic mobility to shift to more negative values, even when the EM is initially above the mineral’s PZC. The only pH region of question regarding this item is in the vicinity of pH 9.
Flotation recovery of chromite as a function of pH at constant SDS concentration 1 x 10 -4 M is shown in figure 2. The four separate curves of the figure demonstrate the effect of aging of dry chromite that had been ground to flotation size (100/150 mesh) on subsequent flotation behavior. The fresh


ground chromite floated well below the mineral’s PZC with a minor drop in flotation near pH 2. The fresh ground mineral did not generally float well with this collector above the mineral’s PZC although there was a small flotation peak near pH 11. Further, in all cases, with or without aging in air the flotation behavior was similar near the mineral’s PZC and there was a dramatic increase in recovery as pH dropped from near pH 5-5.5 to about pH 4. At all other pH values the recovery changed markedly with long aging in air. Above the PZC recovery was little changed after four months aging in air. However, after eight months aging recovery above the PZC was much greater and in general yet slightly greater after twelve months aging. There did appear to be small flotation peaks near pH 7-8 and pH 11, particularly for the twelve months aged material. With air aging below the minerals PZC large dips or troughs appear at pH2 and below (the trough shifting to more acid values and becoming greater with increased aging).
Figure 3 shows the effect of aging in air on the flotation recovery when using 1 x 10 -4 M DAC as the collector. In this system little flotation takes place below the PZC regardless of aging. However, above the PZC flotation increases with aging, particularly after nine months in air.
One set of experiments was performed in which SDS was used as collector (1 x 10 -4 M), chromite was aged in air for twelve months and conditioning time in collector solution was set at one hr. Results obtained are depicted in graphical form in figure 4. Also shown on the figure for comparison is the curve from figure 2 for short conditioning and twelve month aging. It is interesting to note that the additional conditioning reduced flotation throughout the pH range 1-12. Further, the small flotation peaks near pH 8 and 11 were eliminated.
Figure 5 illustrates the effect of mild preheating of chromite on flotation behavior when SDS was used as collector. Shown in the figure is the curve from figure 2 for fresh ground chromite. This curve is compared with one obtained on fresh ground chromite that had been preheated in air at 50°C for 3 hr. A marked change in flotation behavior after preheating is evident. Note the great dip in flotation below pH3 and the enhancement in flotation above the mineral’s PZC, particularly between pH5 and 8 and in the vicinity of pH10. Also, it should be noted that the curve for the preheated chromite is quite similar to that for chromite aged 12 months in air (see figure 2). Thus, preheating appears to greatly accelerate aging kinetics.
The results of the final series of flotation experiments performed are shown in figure 6. In this figure are flotation recovery versus pH curves for fresh ground chromite prewashed in cone HCl for one week. These curves should be compared to those of figures 2 and 3. The prewashing in HCl must remove loosely attached cations and anions adhering to the mineral’s surface giving rise to a “pure” chromite surface. After such treatment, the result is smooth curves without minor ups and downs, good flotation using SDS in the acidic pH region, but poor in the basic region and good flotation using- DAC in the basic region but poor in the acidic region. Such results are rather typical of “physical” adsorption systems where adsorption is controlled by mineral-collector electrostatic charge interaction plus association of hydrocarbon chains (Iwasaki, Cooke and Colombo, 1960, and Fuerstenau and Palmer, 1976).




To study the system further leaching tests were performed using the chromite. In these tests 10 g of -325 mesh chromite (aged in air either 2 weeks or 3 months) were placed in 1 liter of 0.1N HCl. In figure 7 are shown concentrations of Fe, Ca, Mg and Cr as a function of leaching time up to 50 hr. All metals went into solution to at least a limited extent and the amount of all metals going into solution save for Ca increased as a function of time. In general, dissolution of the metals was greater from the longer aged in air material.
Discussion
According to Fuerstenau and Palmer (1976), flotation of a negatively charged mineral using an anionic collector can take place if a hydroxy complex of a heavy metal ion is present over a certain range of concentration of the complex. The concentration of the hydroxy complex is, for a given total concentration of that metal, a function of pH (Butler, 1964, and Fuerstenau and Palmer, 1976). The metal can be added intentionally, can be derived from the mineral to be floated itself or can be derived from other minerals present in the ore. Considering figure 2 and the several curves of the figure, one can assume that the flotation peaks in the vicinity of pH 11 are due to activation of the mineral via the magnesium first hydroxy complex or perhaps the calcium charged hydroxy complex. The peaks near pH 8 on the curves for samples aged in air for long periods of time are probably due to activation via the charged ferrous hydroxy complex. It appears that aging in air (figure 2) and mildly preheating (figure 5) the mineral increases the amount of these metal complexes available for dissolution and adsorption onto the mineral and hence activation of the mineral for anionic flotation above pH 4-5.
The increased availability of the cations would be through breakdown of the mineral’s surface via oxidation of Fe(II) to Fe(III). Some hematite would be formed. In such a case both Fe(II) and Fe(III) species should be more susceptible to dissolution. Also contamination cations, such as Mg(II) and Ca(II), should become more readily soluble through the disturbance. Once in solution the hydroxy complexes of the metals should form and activate the mineral. Alternately the activation can be explained by considering it taking place directly on the surface without dissolution and readsorption. It is probable in such cases that semi-conducting properties of the surface are altered in such a way as to enhance anionic collector adsorption (Sobieraj, Haber, and Laskowski, 1972). The general decrease in anionic flotation (figure 4) with increased conditioning in collector solution must be related to increased dissolution of surface metal species. Thus, they either react in solution with the collector resulting in loss of collector to insoluble metal-collector compounds or their simple absence from the surface reduces adsorption sites for anionic collector adsorption. The increase in cationic flotation with aging of the mineral in air (figure 3) is difficult to explain. Perhaps in this case the increased oxidation of the surface allows for dissolution without reaction with the collector of some of the loosely attached metal oxide and hydroxy species giving the cationic collector more sites on which to adsorb. Again, note the curves of figure 6. Apparently preleaching of the mineral removes easily soluble metal ions from the mineral surface and, thus, the various flotation peaks for the anionic collector above the mineral’s PZC are removed.

Going back to figures 2, 4 and 5 the curious depression of flotation in the acid pH region is more difficult to explain. Obviously it increases with aging of the mineral in air. Sobieraj, Haber, and Laskowski (1972) have studied the effect of annealing chromites in air on their surface properties. These workers found evidence for the oxidation of FeO to Fe2O3 and Cr2O3 to CrO3. Apparently during annealing Cr 3+ is first oxidized to Cr 6+ on the mineral’s surface followed by the reaction:
![]()
Considering Table II it can be determined that the ∆G° 298 value at 298°K for equation (1) is -70.5 kcal. It is also interesting to note the following reactions along with Table II:

From these several relations we observe that in the presence of 1 atm. oxygen pressure chromite is thermodynamically unstable with respect to CrO3, Cr2O3 and Fe2O3 but not FeO. This information should be used with caution because: 1) the relations tell nothing about rate of any of the reactions and 2) the chromite studied was quite impure containing Fe2O3 and impurities. However, the reactions clearly indicate the potential presence of Fe(III) and Cr(VI) species on the surface of chromite.
Once Cr(VI) appears on the surface it can react with water according to the following reactions:

In Table III are listed Gibbs Free Energy of formation values for aqueous Cr(VI) species and selected Fe(III) and Fe(II) aqueous hydroxy species (from Rossini, et.al., 1952). Also listed is the Gibbs Free Energy value for liquid water. All data is for 298°K.

From these data equilibrium constants for reactions 6-8 at 298°K can be calculated as could also similar constants for reactions involving other species listed. Stability constants for equations 6, 7, and 8 are respectively 21.13, 0.184 and 3.95 x 10 -7. What can be deduced from this type of information is the proportion of the various Cr(VI), Fe(III) and Fe(II) species likely to be present in solution as a function of pH. From such data log concentration diagrams such as is depicted in figure 8 can be constructed. According to Butler (1964) when Cr(VI) concentration is 1 x 10 4M or less, dichromate species are negligible. Figure 8 shows that between pH0.74 and pH6.4 (the pH’s of the two pka values for chromic acid) the predominant Cr(VI) species likely to be in solution is HCrO4. Below pH0.74 H2CrO4(aq.) dominates and above pH6.4 CrO4 2- is predominant. Similar diagrams can be constructed for the Fe(III) and Fe(II) systems.
To further study the role of Cr(VI) species, the adsorption of SDS onto the chromite was measured as a function of pH in the absence and, in the acid pH region, in the presence of 5 x 10 -5M CrO4 2-. Figure 9 shows graphically the results obtained. Note that below pH4.5 the presence of CrO4 2- decreased apparent SDS adsorption. It should be kept in mind, however, that in order to obtain the data of this figure the mineral was reacted with the solution for two hours, a much longer conditioning time than was generally used with the flotation experiments.


The behavior of the chromite studied in the acid pH region should be considered, then, in light of the preceding discussion on surface oxidation of Cr(III) and on the makeup of chromate containing solutions. That chromium can be present in solution is evident from the data of figure 7 (up to several mg/l of Cr). In figure 3 it was noted that aging of chromite in air decreased flotation below about pH3 and increased it somewhat between pH3 and 6. Increased conditioning in water decreased flotation in the acid region (figure 4). Preheating in air decreased flotation below pH3, but increased it above this pH value. Washing the chromite in concentrated HCl eliminated the dip in flotation near pH2 and increased flotation between pH4 and pH7.
It appears that below pH3 some one or more chromate species can function as depressants in the chromite system. Oxidizing the surface through aging in air or heating in air should increase the quantity of Cr(VI) on the mineral’s surface. Of interest is the evidence of Davis and Leckie (1980) regarding adsorption of a complex between CrO4 2- and HCrO4- onto synthetic iron oxy-hydroxide in the acid pH range and the findings of Huang and Wu (1977) that HCrO4- is adsorbed onto activated carbon in the acid pH range. However, in the pH region between pH3 and 6 a good part of the Cr(VI) present was reduced in their carbon system to Cr(III) and adsorbed onto the carbon in this form (reaching a maximum at about pH5).
It can be deduced that below pH3 (reaching a maximum at pH1-2) the major specie depressing flotation of chromite is HCrO4-. Below pH0.74 the depressing trend going to more acid pH values is reduced via conversion of some of the HCrO4- to H2CrO4 (aq). It appears that the HCrO4- species is functioning (though in a reverse manner) in much the same manner as Fuerstenau, et.al., 1975, 1976, propose for metal hydroxy species. Apparently in the pH region between pH3 and 6 the Cr(VI) is being reconverted to Cr(III) in the aqueous chromite system (perhaps via reaction with Fe(II) species). Thus, in this pH region anionic flotation is enhanced rather than depressed probably because of the presence of chromic species in solution and the absence of chromate.
If the mineral is strongly acid pretreated all easily soluble surface species (or merely active species) are removed and little activation or depression takes place by either multivalent anions or cations and adsorption of both anions and cationic surfactants appears to be through simple electrostatic interaction between mineral and surfactant plus hydrocarbon chain interaction.
Experimentation on Gangue Minerals Associated with Chromite
The gangue minerals studied were olivine, diopside, bronzite and hedenbergite. Olivines vary considerably in composition from forsterite, Mg2SiO4, to Fayalite, Fe2SiO4, (Deer, Howie and Zussman, 1962a). Diopside, Ca½Mg½SiO3, and hedenbergite, Ca½Fe½SiO3 are monoclinic pyroxenes. Bronzite, Mg0.7-0.9, Fe0.1-0.3 Si03 is an orthopyroxine (Deer, Howie and Zussman, 1962). Chemical analyses of these four minerals are tabulated in Table IV.

In addition, using a scanning electron microscope probe, a trace of phosphorous in the olivine, a trace of chromium in the diopside and a trace of copper in the hedenbergite were detected. Electrokinetic, collector adsorption and a limited number of flotation tests were performed on these minerals. Also, for comparison purposes some electrokinetic and collector adsorption tests were performed on the chromite studied. All these experimental tests were performed as noted previously. In some of the tests various multivalent ions were added.
Electrokinetic Studies
Figures 10 through 13 show the variation of electrophoretic mobility (EM) with pH and as a function of aging time in water for olivine, bronzite, hedenbergite and diopside, respectively. PZC’s (points of zero charge) were determined from these figures (the pH values at the points where the curves crossed the zero EM axis. These PZC values are listed in Table V.
It should be noted that the pH value where the EM versus pH curve crosses the zero EM line is correctly denoted PZC only for a pure mineral in the absence of specific adsorption. If multivalent ions are present in the system, for example, the pH at which such a curve crosses is better denoted point of zero mobility (PZM).

Figures 14 through 18 show the effect of ionic species (cations and anions) on the electrophoretic mobility (EM) of olivine, bronzite, hedenbergite, diopside, and chromite respectively. The corresponding PZM values are presented in Table VI.










Adsorption of Sodium Dodecysulfate (SDS) Onto Chromite Minerals
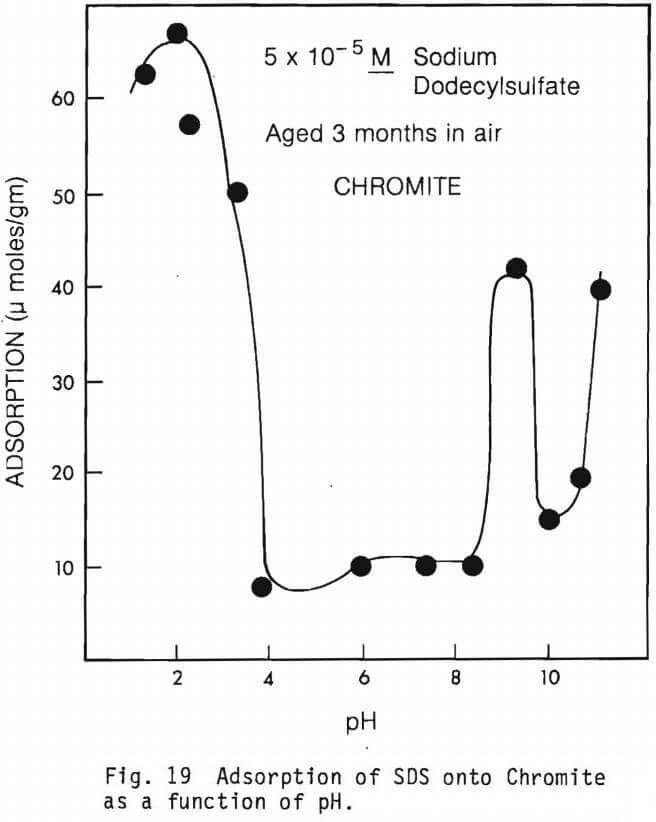
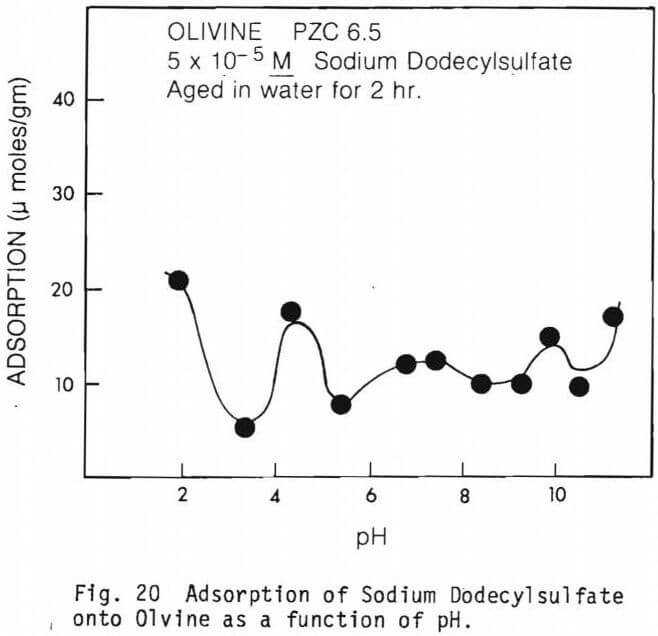
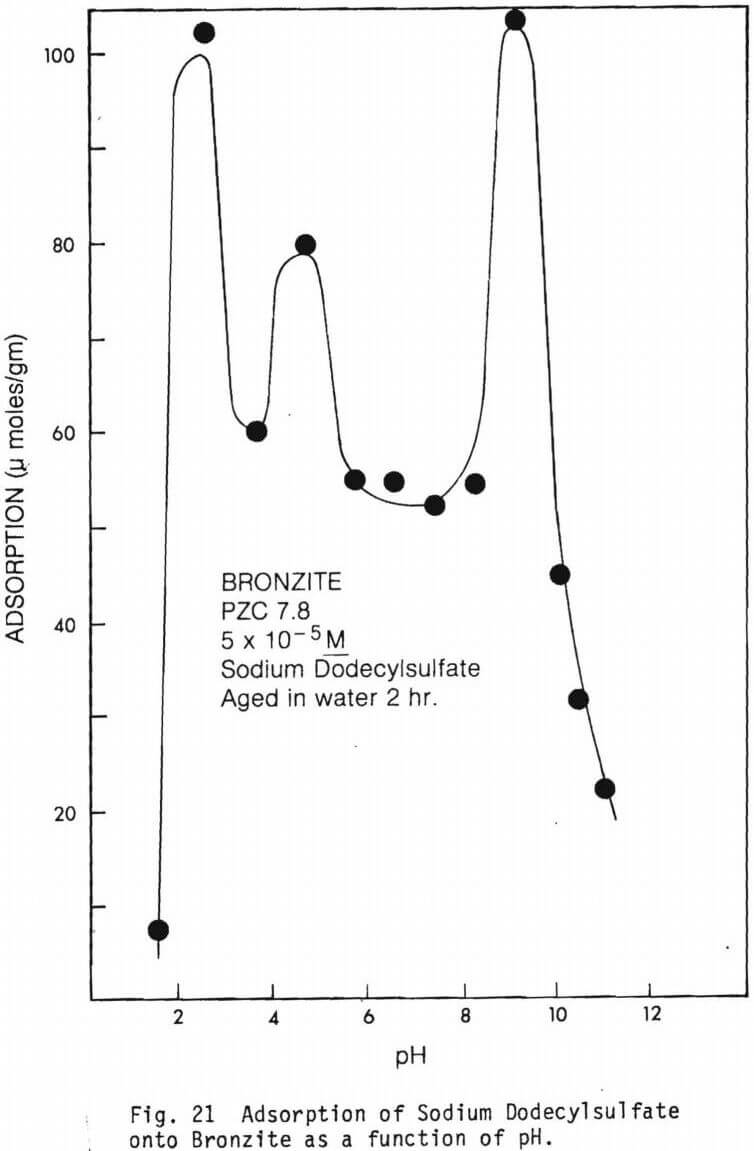
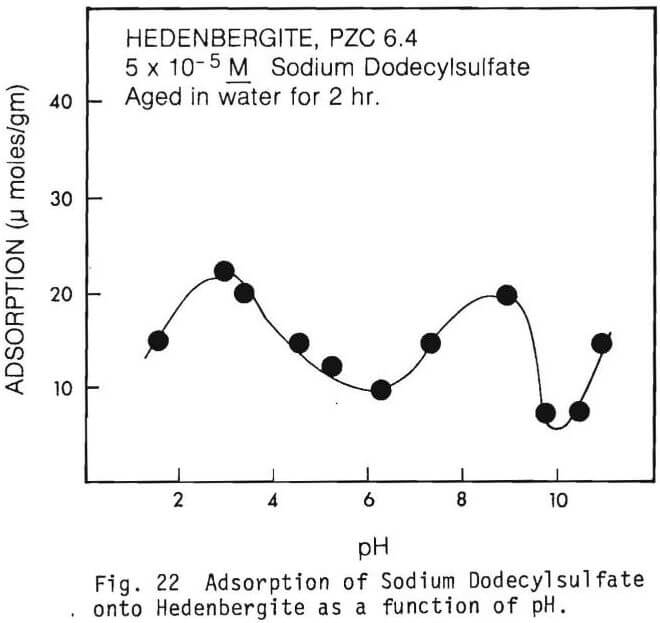
The amount of SDS adsorbed per gram of mineral as a function of pH in the absence of multivalent ions are graphically presented in figures 19 through 22.
Figure 19 shows the SDS adsorption characteristics of chromite which was allowed to age in air for 3 months. The curve of this figure should be compared to that of figure 9 for adsorption of SDS onto fresh ground chromite in the absence of multivalent ions. For the aged chromite it is apparent that adsorption of SDS is much greater in the acid pH region.
Figure 20 represents the adsorption of surfactants on olivine. Relatively low adsorption was observed for olivine at all pH’s.
Relatively high adsorption of surfactant was observed for bronzite at all pH’s and adsorption peaks were registered at about pH 2.6 and pH 9.0. This is indicated by figure 21.
Figure 22 indicates the adsorption of surfactant on hedenbergite. Adsorption was relatively low at all pH’s. However, small adsorption peaks were observed at about pH3.0 and pH9.0.
In addition to the adsorption studies in the absence of added multivalent ions, the effect of addition of Cr(VI) and Fe(II) on adsorption of SDS onto olivine, hedenbergite and diopside were monitored. The bronzite used in the electrokinetic experiments became exhausted and a new bronzite obtained had markedly different adsorption characteristics. Thus, although some adsorption measurements were made using the “new” bronzite the results appear not to be meaningful and cannot be compared with data obtained on the “old” bronzite.
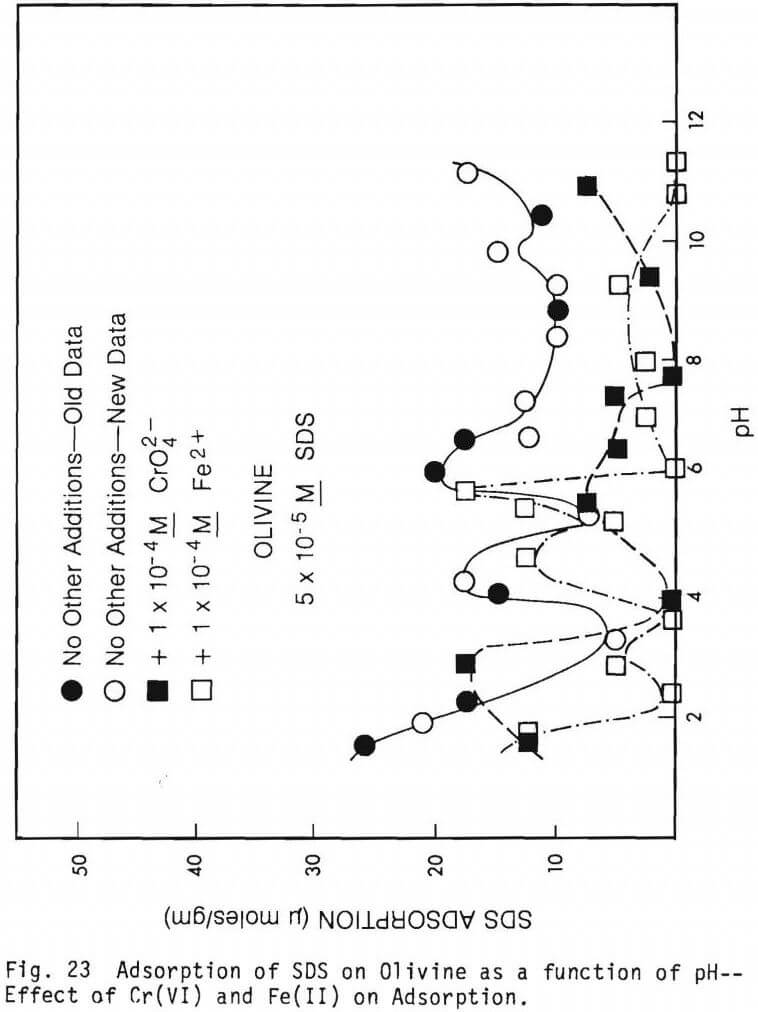
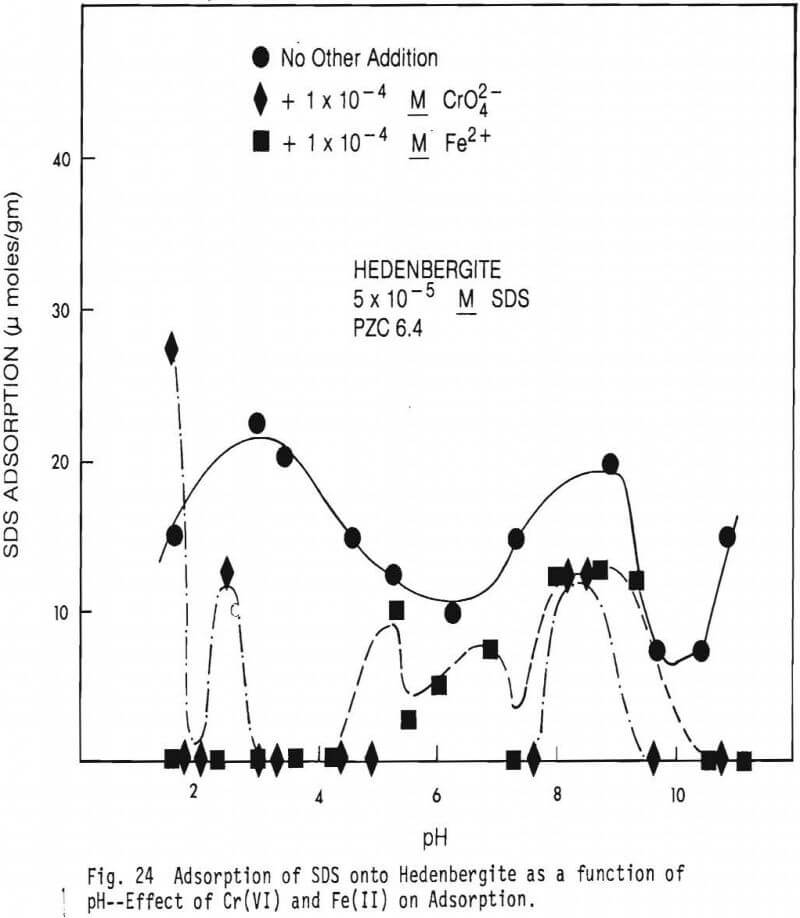
Figure 23 shows curves depicting the adsorption of SDS onto olivine in the presence of Cr(VI), in the presence of Fe(II) and in the absence of both. Figure 24 shows similar curves for hedenbergite and figure 25 shows similar curves for diopside.
Flotation of Gangue Minerals Associated With Chromite
Hallimond tube flotation response of olivine, diopside and hedenbergite was measured as a function of pH using SDS as collector in the absence of added multivalent inorganic ions and in the presence of Fe(II). Figure 26 shows flotation recovery data obtained on the olivine studied. Two different


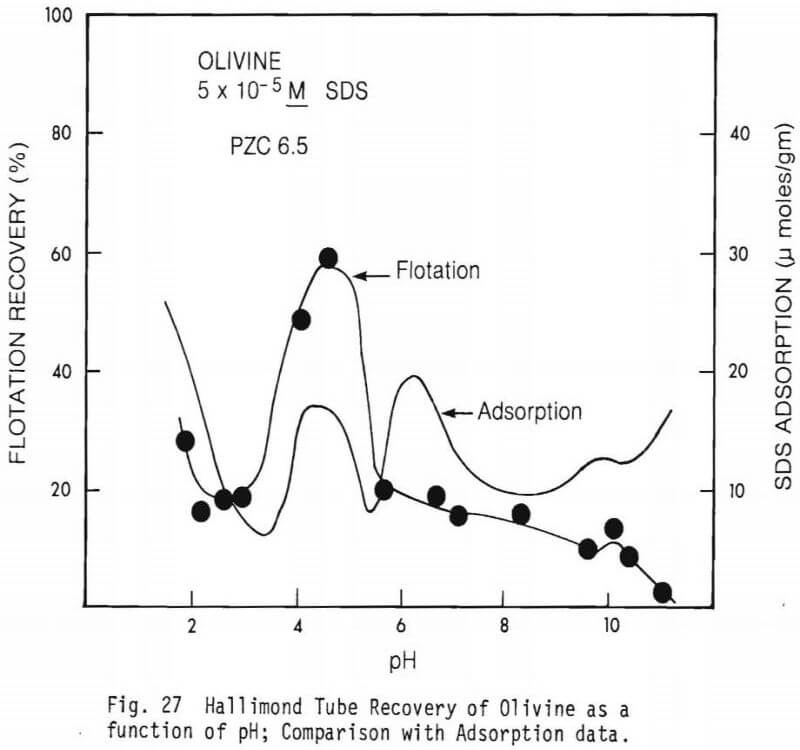
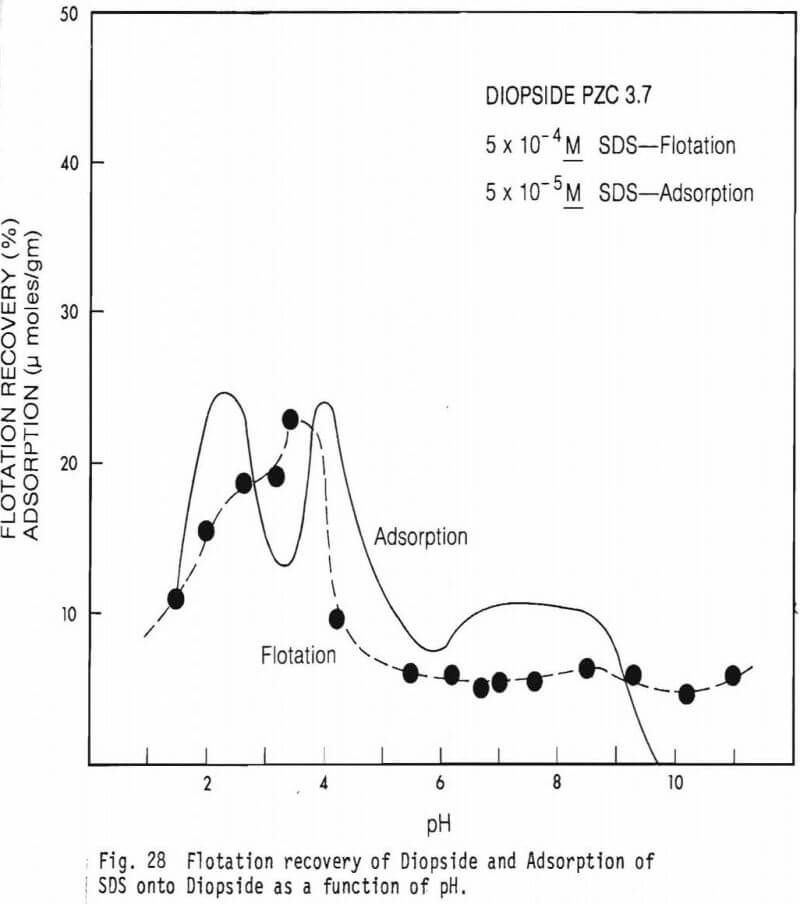
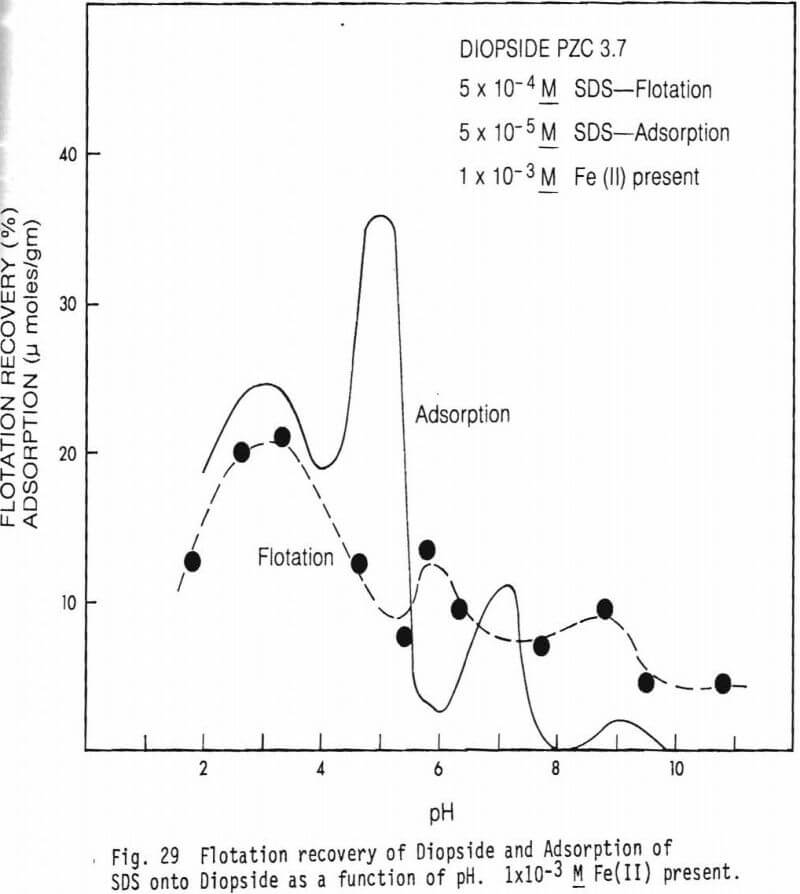
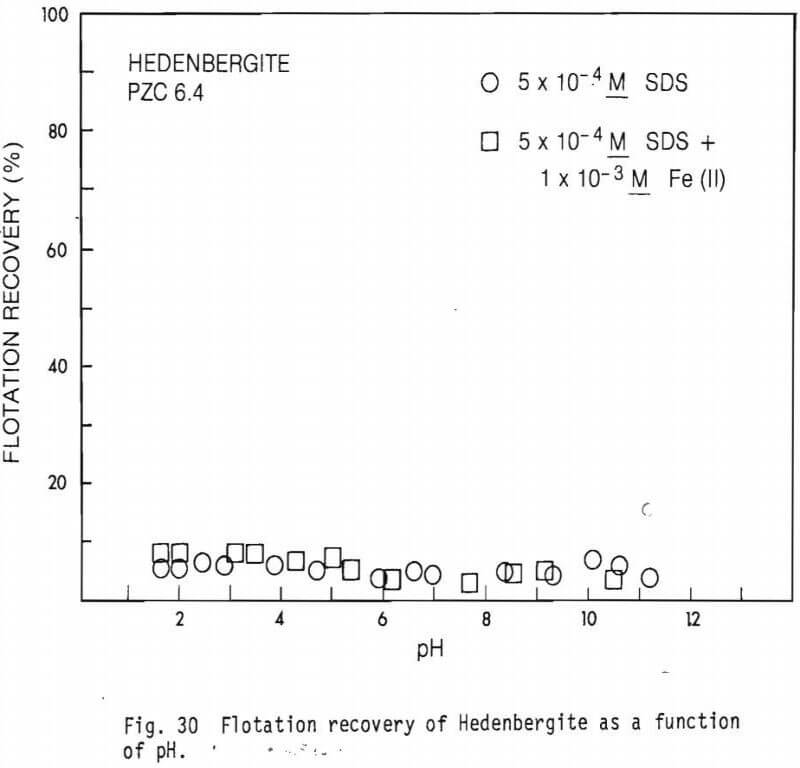
collector concentrations, 5×10 -5M SDS and 5×10 -4M SDS were used. Data on the effect of Fe(II) on flotation recovery is also shown (collector concentration 5×10 -4M). Figure 23 shows a comparison of flotation recovery curves when using 5×10 -5M SDS as collector to SDS adsorption data. These curves are for figures 23 and 26. Figure 28 shows Hallimond tube flotation recovery data for the diopside studied as a function of pH when 5×10 -4M was used as collector. For comparison an adsorption curve from figure 25 is also shown. Figure 29 presents similar data to figure 28 except that 1×10 -3MFe(II) was also present in the system. Figure 30 shows flotation recovery of the hedenbergite as a function of pH using 5×10 -4M SDS as collector and in the presence and absence of Fe(II).
Gangue Minerals Performance Review
Figures 10, 11, 12, and 13 show the variation of electrophoretic mobilities with aging time in water, and as a function of pH for olivine, bronzite, hedenbergite and diopside respectively. These figures directly indicate the pH dependence of surface charge of the above silicate minerals. For these minerals, hydrogen and hydroxyl ions are potential determining. Adsorption- dissociation of H+ from surface hydroxyls accounts for the surface charge on the minerals. Surface charge has long been attributed to the amphoteric dissociations of surface MOH group, and the adsorption of metal hydroxo-complexes derived from the hydrolysis products of materials dissolved from the mineral (Parks, 1965 and Parks, 1967). The dissociation reactions can be represented as follows:

Acid dissociation (or negative adsorption of H) produces negative surface sites; and basic dissociations (or positive adsorption of H+) produces positive surface sites. The formation of surface charge by above mechanisms would result in a change of pH of the solution.
Table V and figures 10, 11 and 13 show the PZC’s of olivine, bronzite, and diopside to shift to a more basic pH with aging time in water. This basic shift in pH can be attributed to the dissolution of disturbed layers on the mineral’s surface, accompanied by later re-adsorption of metal hydroxy species back onto the surfaces (Smith and Trivedi, 1974). The lowering of the PZC of bronzite after two hours aging (Table V) can, therefore, be attributed to the dissolutions of a disturbed layer on the surface without the readsorption of the metal hydroxy species.
The PZC of hedenbergite shifts to more acid values with aging time in water (Table V and fig. 12). This phenomena may be due to loss to solution of soluble cations whose oxides possess basic PZC’s. The disturbed layers on these mineral surfaces result during the sample preparations (grinding) as illustrated earlier in the case of quartz.
Surface phenomena in silicate minerals can also be related to the oxygen-silicon ratio in the silicate structure. Deju and Bhappu (1972) showed that the adsorption of hydrogen ions by the mineral surface increases as the oxygen-silicon ratio increased. For example, olivine will adsorb more hydrogen ions than quartz. A proportionality is observed between the oxygen-silicon ratio and the PZC of the mineral. The reaction between silicate mineral particles and water involves mainly an exchange of metals ions for hydrogen ions, leading to an increase pH of the aqueous solutions.
Note from Table V, the low value of the PZC of diopside compared to the rest of the minerals studied. Diopside is a pyroxene similar in structure to hedenbergite and bronzite, and yet there are great differences between their PZC’s. The only possible explanation for the behavior of diopside is the presence of large amounts of calcium (17.2% from Table IV) in the structure. It is possible that calcium-oxygen bonds are harder to break than magnesium-oxygen bonds or iron-oxygen bonds in the other structures. This would result in a less positive surface on the diopside and less adsorption of hydroxyl ions from solution and a more acid pH of the PZC.
An alternative explanation could be that calcium hydroxide is more soluble than magnesium hydroxide and hence, more calcium ions will be lost to solution as the hydroxide, resulting in a more acid PZC.
Effect of Ionic Species on Electrokinetic Behavior
Figures 14, 15, 16, 17, and 18 show the effect of the presence of some ionic species on the PZC’s of olivine, bronzite, hedenbergite, diopside and chromite respectively. It is noted in figure 14 that CrO4 2- tends to lower the PZM of olivine while the multivalent cations tend to raise it. An exception seems to be Fe2+ which changes the EM curve relatively little. In the case of bronzite, the anionic substance, CrO2 4-, and the cations Fe2+, and Fe3+, tend to lower the PZM while Mg2+, Cr3+, Al3+ tend to raise the PZC (refer to figure 15). The PZM of hedenbergite is lowered by CrO4 2-, and Mg2+ while Fe2+, Fe3+, Cr3+ and Al3+ tend to raise it (refer to figure 16). The effect of CrO4 2- on the PZC of diopside cannot be determined from the EM curve (figure 17) because of insufficient data. Moreover, Mg2+ tends to lower the PZM of diopside while Al3+, Cr3+, Fe2+ and Fe3+ tend to raise it (figure 17).
Above data indicates that generally the anionic substances would tend to shift the PZM’s toward more acidic values while the cationic species would tend to shift them toward more basic pH’s. These phenomena have been attributed to specific adsorption of the ionic species. According to Overbeek (1952) the specific adsorption of anions would be expected to produce a negative surface charge and more acid hence, the PZM would be shifted to a more acid pH because increased H+ adsorption would be required to neutralize the anion’s negative charge. According to Fuerstenau and Palmer (1976), polyvalent metal ions also adsorb specifically on oxides and silicates. This phenomenon occurs when the cation involved hydrolyzes to its first hydroxy complexes.
This general behavior of cationic substances, however, does not explain the lowering of PZC by Mg2+ in diopside and hedenbergite; and by Fe2+ in olivine and bronzite; and by Fe3+ in bronzite. The formations of hydroxy complexes would be the more plausible explanation of the observed behavior. Hydroxy complexes are such surface active species that they will adsorb on a positively charged surface (Fuerstenau, Elgillani and Miller, 1970).
The form of the cationic species present in solutions at different pH values are presented in figures 31, 32, 33 and 34 (from Fuerstenau and Palmer, 1976) for Fe2+, Mg2+, Fe3+ and Al3+ respectively. Their effect on adsorption of an anionic collector would be as noted earlier.
Figure 18 shows the effect of the presence of Cr3+, CrO4 2- and Fe2+, on the electrokinetic behavior of chromite. The PZM is lowered by CrO4 2- while Fe2+, and Cr3+ tend to raise it. According to Pourbaix (1966) CrOH2+ is the most significant Cr (III) species between approximately pH 4 and pH 6 and Cr(OH)2 is predominant between pH 6 and pH 8 . These species appear to be the surface active ions causing EM shift in the system.
Analysis of the minerals used in this study (Table IV) indicate the presence of calcium, magnesium, iron and aluminum. Traces of chromium and copper were also detected using the scanning electron microscope (SEM). Hence, it is possible to suggest that these cations and their hydrolysis products, whether present as constituent ions or as impurities, are partly responsible for observed behavior in aqueous solution.
Adsorption Studies on Gangue Minerals
The adsorption of sodium dodecyl sulfate (SDS) collector on olivine is shown in figure 20. Note in this figure that the adsorption of SDS on olivine is relatively constant over the pH range studied except for the low peaks at about pH 2.0, pH 4.0-5.0, pH 10.0 and pH 11.0. Physical forces seem to be relatively unimportant in the adsorption of SDS on olivine except around pH 5.0 and below.
The PZC of olivine as determined from electrokinetic study was 5.85. Note the depression between pH 3 and 4. This behavior might be due to some contaminant ion or other coatings on olivine. One possibility is the presence of trivalent ions, Cr, Fe and Al in above pH range. Paik (1967) conducted infrared and flotation studies of the adsorption of sodium alkyl benzene sulfonates on olivine and postulated a possible mechanism for the attachment of RSO3 (sulfonate radical) to the olivine surface.
Mg(OH)2(S)+RSO3 = Mg(RS03)2(S)+2OH-
That is, collector anions adsorb by exchange with OH- formed on the surface by surface hydration of broken bonds. Hence, sodium dodecyl sulfate will probably behave similarly in aqueous solutions. According to Deju and Bhappu (1972), however, for sulfonates with 12 carbon atoms or less in the chain, the solubility is too high for any hemimicelle to form but for carbon chains longer than C12,Mg(RSO3)2 is soluble enough for hemimicelles to form and for flotations to occur. A hemimicelles is an aggregation of the hydrophobic groups, which may occur near a surface at concentrations well below the critical micelle concentration of the surfactant in the bulk of the solution.
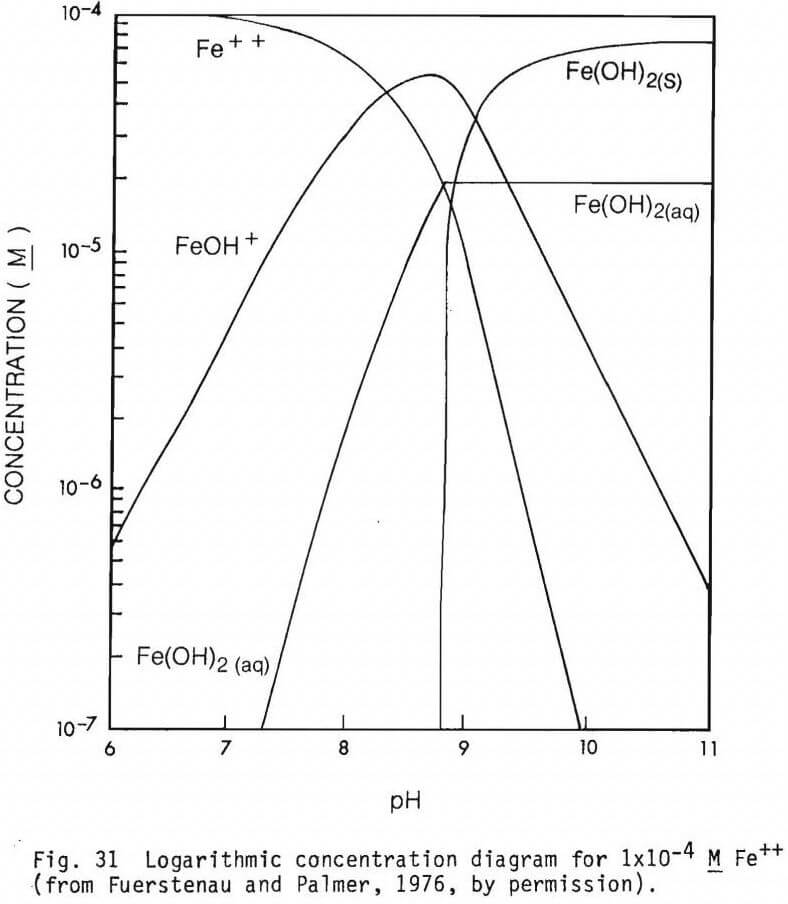
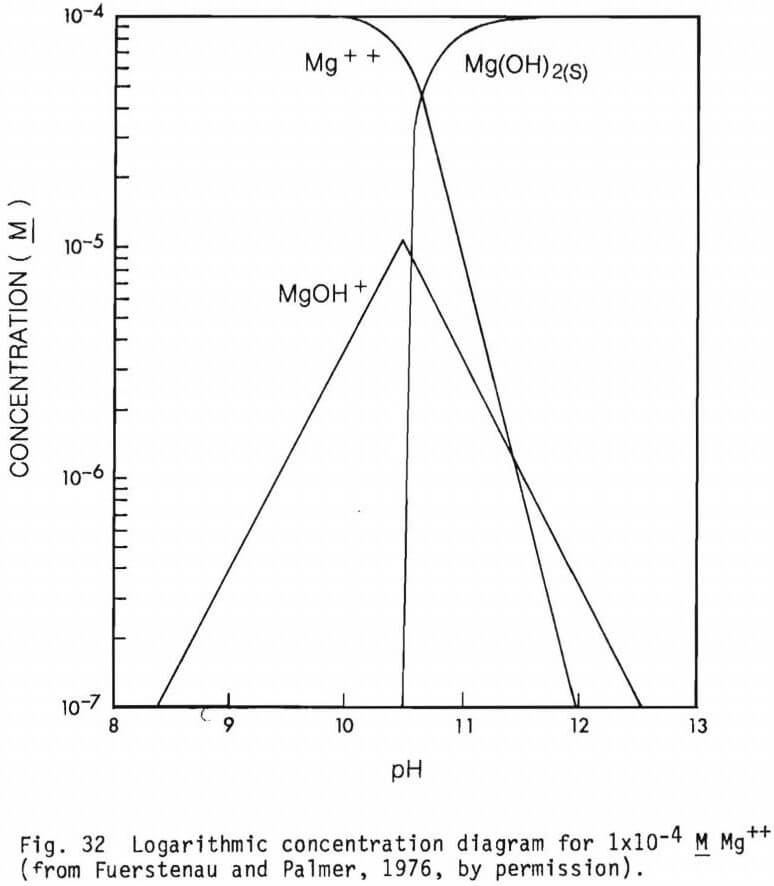
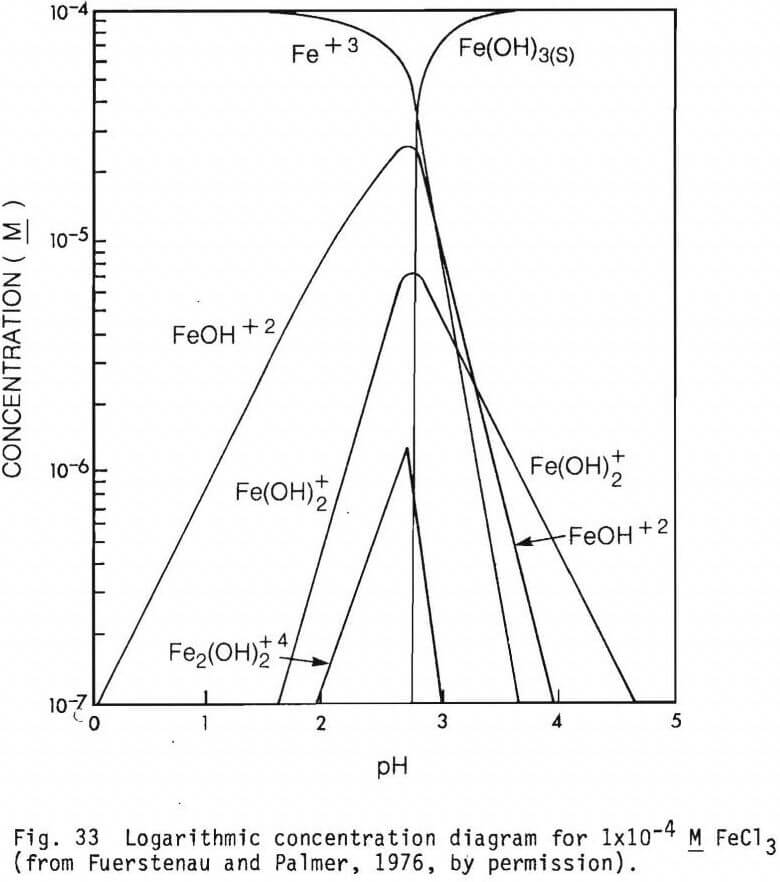
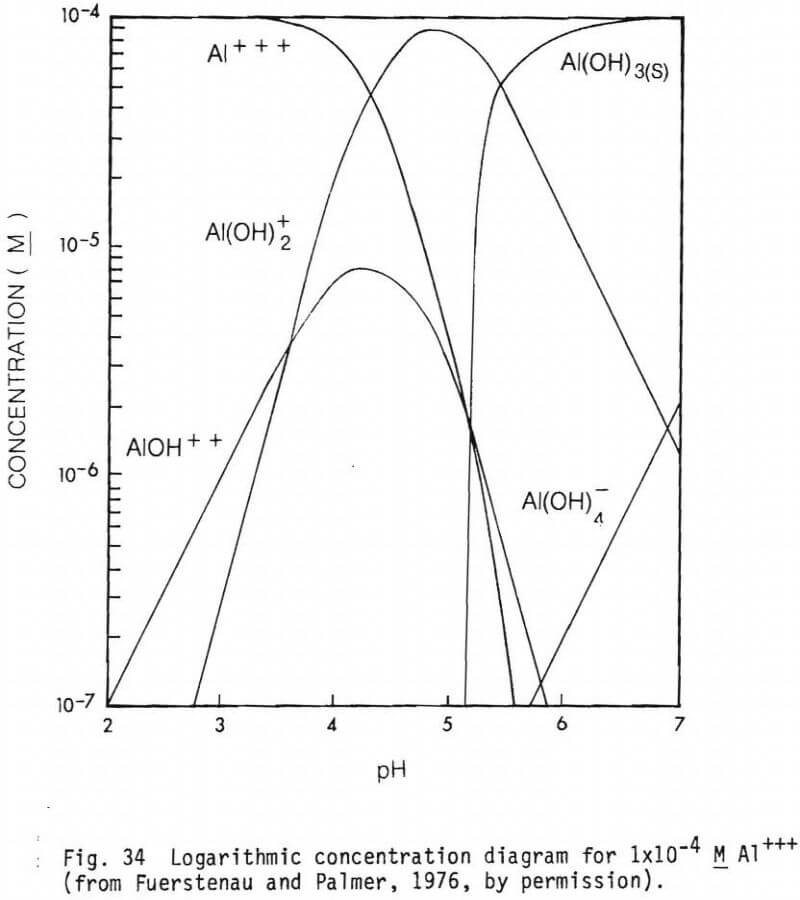
Figure 21 shows the adsorption of sodium dodecyl sulfate on bronzite. Adsorption is relatively high at all pH values studied with high peaks around pH 2.0 and 9.0, and a lower peak between pH 4 and 5. The PZC of bronzite is between 5.9 and 7.4. Within this pH range, SDS adsorption is relatively low and is higher below and above this range. Physical adsorption forces seem to predominate below the PZC and chemisorption through the formation of metal ion complexes seem to be acting above the PZC. Note the depression between pH 3 and 4. As in olivine, trivalent cations, Cr3, Al3+ and Fe3+ may be responsible for the depressing action. Above the PZC, Mg(II) species are predominant between pH 8 and 10. Thus, both physical and chemical forces seem to be responsible for SDS adsorption on bronzite.
Figure 22 shows the adsorption of 5×10 -5M SDS on hedenbergite. The PZC of hedenbergite as determined from electrokinetic studies is between 5.0 and 7.4. Note the adsorption peaks above and below the PZC. Adsorption below the PZC is probably due to physical interactions between the positively charged mineral surface and the negatively charged collector anion, RSO4. Above the PZC, specific adsorption of ionic species, such as Ca2+,Mg2+, and their hydrolysis products seem to be responsible for collector adsorption. Note the depression near the pH of the PZC. This can be explained by the fact that the mineral surface becomes less positive between pH 5.0 and 7.4, thus, physical forces and attraction between collector and the mineral are reduced. Another possibility might be the presence of Aluminum species, such as Al(OH)4 (fig. 34).
Effect of Multivalent Ions on Adsorption of SDS onto Gangue Minerals
Figure 23 depicts adsorption of SDS onto olivine in the presence of Cr(VI) and Fe(II) aqueous species. For comparison, a curve showing adsorption of SDS onto olivine in the absence of such multivalent ions is also shown on the figure. Obviously all the ups and down of the various curves are confusing and difficult to interpret. It does appear that the presence of Cr(VI) over most of the pH range studied inhibits the adsorption of SDS onto the mineral. Fe(II) also in general inhibits the adsorption of SDS onto olivine. While the action of Cr(VI) can be explained on the basis of competition between CrO4 2- and HCrO4- ions and dodecylsulfate ions for adsorption sites on the mineral the apparent depressing action of Fe(II) species is unknown at present. It can be noted from figure 14 that both Cr(VI) and Fe(II) additions changed the electrokinetic behavior of olivine very little.
Figure 24 shows hedenbergite data similar to that of Figure 23 for olivine. The point of zero charge (PZC) of the hedenbergite studied is near pH6.4 and the mineral contains a substantial amount of iron (see Table IV). Thus, it is not surprising that in the absence of added CrO4 2- or Fe there is considerable adsorption of SDS below the mineral’s PZC and an adsorption peak near pH8.5-9.
The former is probably related to coulombic interaction between mineral and collector and the latter is probably related to the presence of substantial quantities of FeOH+ in solution near pH8.5-9. Addition of CrO4 2- drastically lowered the mineral’s point of zero mobility to a pH value less than 4 (see figure 16). It is, therefore, to be expected that the addition of Cr(VI) to the system would reduce SDS adsorption at all but quite acid pH values which is what takes place. There, of course, remains an adsorption peak near pH8.5 which is somewhat reduced in size. The adsorption data, however, for the addition of Fe(II) is not consistent with the measured effect of Fe(II) on the electrophoretic mobility (EM) and PZC of hedenbergite (figure 16). The presence of Fe(II) shifted the EM generally to more positive values, and, thus, SDS adsorption should have been increased by the addition of Fe(II). Such apparently is not the case since SDS adsorption decreased at all pH values with such addition.
Figure 25 shows similar data on diopside. From Table IV it is to be noted that the PZC of the diopside studied is at pH3.7. The mineral contains much Ca and Mg, but little Fe. In the absence of introduced Fe(II) or Cr(VI) an adsorption peak appears below the PZC as is to be expected. Also there is an adsorption peak near pH4. The reason for the existence of this peak is not known. Considering the curves of figure 25 for the cases of addition of CrO4 2- and Fe2+ the adsorption peaks below the mineral’s PZC are reasonable. However, the ups and downs of the curves at pH values more basic than the PZC cannot be accounted for at present.
Flotation Studies on Gangue Minerals
Hallimond tube flotation studies on chromite ore gangue minerals were performed. The particular minerals studied were olivine, diopside and hedenbergite. The initial flotation conditions are indicated in Table VII.

Under such conditions, although less than expected, olivine flotation was satisfactory. However, the maximum flotation recovery obtained for diopside under such conditions at any pH value was 7.1% (at pH 2.6). For hedenbergite the maximum recovery was 3.1% (at pH 6.5). Thus, in subsequent experiments collector concentrations were increased to 5×10 -4M SDS and flotation recoveries of all three minerals were measured as a function of pH at this collector concentration. In addition to the tests conducted in the absence of inorganic additives flotation tests on all three minerals were conducted in the presence of Fe(II).
Figure 26 shows flotation recovery of olivine as a function of pH in the absence and presence of Fe(II) using the collector concentration of 5×10 -4M. Also shown on the figure for comparison are the flotation data for a collector concentration of 5×10 -5M (no Fe(II) present). It can be seen that the flotation recovery is markedly increased when the collector concentration is increased, especially in the acid region below the mineral’s point of zero charge (PZC). The presence of Fe(II) little affected flotation recovery.
Figure 27 shows a comparison of flotation and adsorption data on olivine. It is to be noted that the correlation is very rough, the best correlation being at pH values less than pH6. It is possible that the poor correlation results from 1) some of the reported “adsorbed” collector being actually collector that had been precipitated and/or 2) the possibility at different pH values the mode of attachment of the collector to the mineral surface differs and is less effective at promoting flotation (at some pH values).
Figures 28 and 29 show flotation recovery of diopside as a function of pH in the absence and presence of Fe(II), respectively, using a collector concentration of 5×10 -4M. For comparison adsorption curves from figure 25 with and without Fe(II) addition are shown on the respective figures. Surprisingly, flotation recovery is much poorer than for olivine. There is a flotation “peak” near pH3 which is slightly below the mineral’s PZC. The presence of Fe(II) affected flotation relatively little. Apparently there is little flotation naturally promoted in the system via multivalent metal activators derived from the system and the addition of Fe(II) failed to effectively activate the mineral even in the pH region (near pH 8.5) of maximum concentration of the Fe(OH)+ specie.
Adsorption only very roughly corresponds to flotation. Although the reasons for the lack of good correlation between adsorption and flotation is not known for certain, one possible reason has to do with differences in conditioning time. For the adsorption tests, in order to set up equilibrium or near equilibrium conditions, a two hour contact of mineral samples in the collector solutions was maintained before analytical measurements were made. The mineral samples were conditioned in the collector solution for only 5 minutes before the flotation experiments were begun. Thus, it seems unlikely that anything approaching equilibrium in solution was present during actual flotation.
Figure 30 shows flotation recovery of hedenbergite as a function of pH in the absence and presence of Fe(II) using a collector concentration of 5×10 -4M SDA. Very little flotation was observed at any pH value. This result was also quite surprising. Adsorption tests had indicated that better flotation at least at some pH values should exist.
Conclusions
The flotation of naturally occurring chromite was found to depend on whether the ground material was aged in air or not and whether the mineral had been heated. In general the observed effects could be attributed to oxidation and reduction reactions involving surface chromium and iron species. Pretreating the mineral with hydrochloric acid appeared to remove altered, easily soluble, surface material. Thus, after such pretreatment the mineral behaved much like a simple oxide with anionic flotation possible only at pH values more acid than the mineral’s PZC and cationic flotation possible only at pH values more basic than the mineral’s PZC.
It appears, then, that the manner in which the chromite ore is treated prior to flotation is of substantial importance in subsequent flotation. Even how long the ore has been stockpiled prior to flotation is likely to be of importance.
Among the gangue minerals studied (olivine, diopside, hedenbergite, bronzite) it appears that olivine is the most readily floated and the mineral most likely to give trouble in a chromite flotation system. However, all the minerals appear to be affected in their electrokinetic and, to some extent in their adsorptive and flotation behavior, by metal ions almost certain to dissolve naturally from a chromite ore. How readily these ions go into solution is likely to depend on past treatment of the ore. Thus, again, aging the ore in a stockpile will probably change its flotation characteristics. Also, it is likely if such metals are present that a suitable depressant acting on such ions should be added to the flotation system.
